Ponto de Fusão e Ebulição - diferentes substâncias em diferentes estados físicos
273.47k views369 WordsCopy TextShare

O Incrível Pontinho Azul
Olá pequenos cientistas! Falamos sobre os estados físicos da matéria e suas transformações, mas as s...
Video Transcript:
Hello, little scientists, I am Professor Bill Tyson and together we are going to know different forms of science. In our last meeting we talked about the physical transformations of matter. Today we are going to talk about the temperature at which the solid substances become liquid and the liquid substances become gaseous.
In nature we find several types of substances in different states, for example the oxygen we breathe is in the gaseous state. The water we drink is in the liquid state and a metal fork that we use to eat is in a solid state. But why are they in different states if they are in an environment under the same temperature?
We call this the melting point, and the boiling point. The boiling point is when a substance passes from the liquid to the gaseous state, it is called so because when the liquid is very heated it starts to make bubbles, then boiling. Each chemical element has its property, its melting point and boiling point.
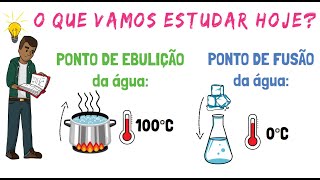
That is, a specific temperature changes the state of matter of a solid, liquid or gaseous substance. Water for example. For it to turn ice, it must be at a temperature of zero degrees celsius.
This temperature is then the melting point of the water. For the water to turn gas, steam must be at a temperature of 100 degrees celsius. And this temperature is the boiling point of the water.
If a substance is found in nature in the gaseous state, it will need a much lower temperature to pass into the liquid state. Oxygen for example, its melting point is 218 degrees Celsius below zero, or 218 degrees Celsius negative. This is the temperature required for oxygen to turn liquid.
And in the case of a substance that we find in nature in the solid state? What is the temperature to become liquid? Iron, for example, has its melting point at 1538 degrees celsius.
Wow, that's pretty hot, huh? Just thinking of this heat all gave me to thirst. For now, it's just personal, see you next time.
How about making science education reach out to more kids? Sign up for the channel and share this video.
Related Videos

10:58
Ponto de fusão e ponto de ebulição - Brasi...
Brasil Escola Oficial
230,277 views

7:01
ESTADOS FÍSICOS DA MATÉRIA: as transformaç...
Toda Matéria
226,357 views
10:15
PONTO DE FUSÃO E EBULIÇÃO
WM Videos Animados
2,661 views

6:27
PROPRIEDADES DA MATÉRIA: ponto de fusão e ...
Curso Enem Gratuito
18,300 views

11:42
Ex-CIA Director predicts chilling effects ...
MSNBC
236,493 views

27:12
Authorities reveal cause of death for acto...
ABC 33/40
1,610,714 views

17:56
🧪 ESTADOS FÍSICOS: TEORIA E EXERCÍCIOS
Professor Gabriel Cabral
55,354 views

8:19
OS ESTADOS FÍSICOS DA MATÉRIA E SUAS TRANS...
Resumos Animados
86,110 views

17:42
ESTADOS FÍSICOS DA MATÉRIA E MUDANÇAS DE E...
Olhar Químico
366,343 views

33:49
Forging a Knife & Survival Camping Alone i...
Outdoor Boys
1,471,459 views

3:06
Evaporação da Água - moléculas ao vento
O Incrível Pontinho Azul
57,917 views

18:07
Canada Just Dealt Russia a CRUSHING Blow!
PPR GLOBAL
257,653 views

27:09
I tested the FIRST EVER limited Edition iP...
Mrwhosetheboss
1,271,201 views

2:33
Mudança de Estado Físico da Matéria - um n...
O Incrível Pontinho Azul
410,539 views

4:17
As mudanças de estado da matéria - Fusão, ...
Smile and Learn - Português
652,947 views

13:48
CURVAS DE AQUECIMENTO - SUBSTÂNCIAS E MIST...
Quimicapontocom - Prof. Zanin
48,930 views

28:34
REVISÃO QUÍMICA BÁSICA I - MATÉRIA, PROPRI...
Café com química - Prof Michel
130,228 views

23:41
GIRLS CAN'T SPEAK At New School | Dhar Man...
Dhar Mann Studios
1,527,889 views

10:46
Os Estados Físicos da Matéria - Brasil Escola
Brasil Escola Oficial
222,907 views

10:02
I Transformed an Abandoned Turtle’s Home
TerraGreen
399,371 views