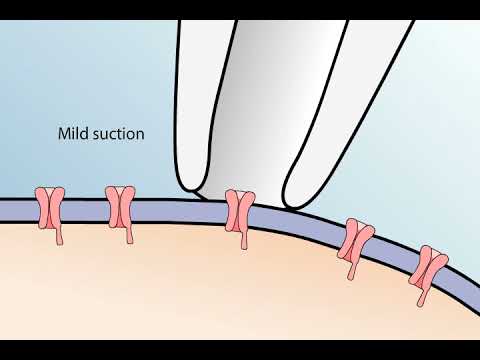
the patch-clamp method is used to study the properties of a small patch of membrane in this technique a glass pipette with a very small opening is used to make tight contact with a tiny area or patch of neuronal membrane after the application of a small amount of suction to the back of the pipette the seal between pipette and membrane becomes so tight that no ions can flow between the PI pad and the membrane thus all the ions that flow when a single ion channel opens must flow into the pipette the resulting electrical current though small
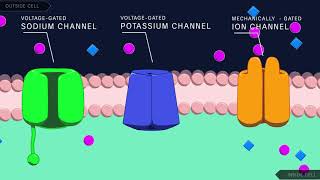
can be measured with an ultra sensitive electronic amplifier connected to the pipette this arrangement is the cell attached patch clamp recording method a record of the current flowing through a single ion channel reveals when the channel is in an open or closed State the currents flowing through single channels are called microscopic currents to distinguish them from the macroscopic currents flowing through a large number of channels distributed over a much more extensive region of surface membrane the technique can be used to compare the response characteristics of different channels for example depolarizing voltage pulses applied to a
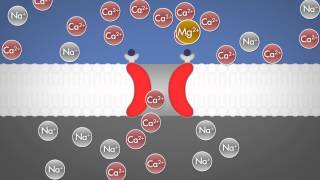
patch of membrane containing a single sodium channel result in brief currents the downward deflections the sum of many such current records show that most channels open in the initial one to two milliseconds following depolarization of the membrane the process by which sodium channels closed is called inactivation when the membrane potential is depolarized voltage sensors indicated by the plus symbol allow the channel gates to open and current flows in word after less than one millisecond the channel in activates when an inactivation gate swings up and blocks the poor the poor remains blocked even during prolonged depolarization
the poor then closes and the inactivation gate opens as the membrane potential returns to resting levels a macroscopic current measured from another axon shows the close correlation between the time courses of microscopic and macroscopic sodium currents reflecting the opening closing and inactivating of channels most channels open in the initial 1 to 2 milliseconds following depolarization of the membrane after which the probability of channel openings diminishes because of channel inactivation technical modifications have enhanced the patch clamp technique for example if the membrane patch within the pipette is disrupted by briefly applying strong suction the interior of
the pipette becomes continuous with the cell cytoplasm this arrangement allows measurements at electrical potentials and currents from the entire cell and is therefore called whole-cell recording two other variants of the patch clamp method originate from the finding that once a tight seal has formed between the membrane and the glass pipette small pieces of membrane can be pulled away from the cell without disrupting the seal simply retracting a pipette that is in the cell attached configuration causes a small vesicle of membrane to remain attached to the pipette by exposing the tip of the pipette to air
the vesicle opens to yield a small patch of membrane with its former intracellular surface exposed this arrangement called the inside-out patch recording configuration allows the measurement of single channel currents with the added benefit of making it possible to change the medium to which the intracellular surface of the membrane is exposed alternatively if the pipette is retracted while it's in the whole cell configuration a membrane patch is produced that has its extracellular surface exposed this arrangement called the outside out recording configuration is optimal for studying how channel activity is influenced by extracellular chemical signals such as
neurotransmitters this range of possible configurations makes the patch-clamp method an unusually versatile technique for studies of ion channel function