Chapter 1: Chemistry Part 1
35.98k views16556 WordsCopy TextShare
Dr. Julie Wells
This video is an introduction to chemistry for Microbiology (Bio 210) and General Biology (Bio 100) ...
Video Transcript:
in this lecture we are going to cover chemistry for biology and you might wonder why are we learning about chemistry in a biology class and what you need to understand is that chemistry is fundamental to biology because cells which are the fundamental unit of life are simply a collection of chemical reactions and so we have to understand some chemistry in order to understand how life works so we're going to start out this lecture by talking about the structure of atoms chemistry is the study of interactions between atoms and molecules matter can be defined as anything
that takes up space and has mass and the fundamental unit of matter is the atom so if we look at an atom there are two main parts to the atom we have the nucleus which is going to be in the center of the atom and orbiting around it we have our electron shells and so in the nucleus of the atom that's where you're going to find particles called protons or neutrons protons carry a positive charge neutrons are neutral or no charge and around the nucleus again in the electron shells this is where you're going to
find the electrons and electrons carry a negative charge so if i look at this hydrogen atom notice that it has one proton and one electron the positives and the negatives cancel and this atom has an overall no net charge so when we talk about atoms that have no net charge what that tells you is that the number of protons is equal to the number of electrons because again protons are positive electrons are negative and so let's talk more about the atom an element is any substance that cannot be reduced to any simpler set of constituent
substances through chemical means each element is defined by the number of protons in its nucleus for example hydrogen always has one proton in its nucleus oxygen has eight carbon has six for an element you can't change the number of protons and have the same element if you change the number of protons you change the element and so that is something that cannot be changed now life requires 25 essential elements the main four that make up about 96 of the human body would be oxygen carbon hydrogen and nitrogen and so if you look these four elements
make up 96 of the human body now there are elements that make up about four percent we have calcium calcium plays a role in muscle contraction we have phosphorus plays a role in certain macromolecules potassium sodium those play an important role in electrical impulses in neurons we have sulfur which is found in proteins we have chlorine which regulates salt balance we have magnesium you'll notice at the bottom we have our trace elements trace elements are required in very small amounts while they're only required in small amounts they are still necessary so some examples of trace
elements that we need to prevent disease one example would be iron iron is important because it is part of a molecule called hemoglobin hemoglobin is a protein in your red blood cells and that protein's job is to pick up oxygen when the blood circulates to the lungs and the oxygen is going to bind to the hemoglobin bind to the iron and it's going to allow hemoglobin to carry oxygen throughout the body because all the cells in your body need oxygen to make atp so if you don't transport oxygen effectively your cells are not going to
make enough atp this results in a condition known as anemia so if you've ever heard that somebody is anemic what that means is they have a problem with their hemoglobin it's possible that they don't have enough red blood cells circulating maybe they've lost blood for example and if they don't have enough blood they don't have enough hemoglobin and therefore they don't transport oxygen effectively and one of the treatments for anemia is sometimes an iron supplement doctors will prescribe an iron supplement because your cells can't make hemoglobin without the iron so you have to take an
iron supplement which would help produce more hemoglobin and therefore transport oxygen effectively and so that is an example of a trace element that you do still need another trace element is going to be iodine iodine deficiency prevents the production of thyroid hormones your thyroid is a gland in your neck and if you have a deficiency in iodine you get what's known as a goiter the thyroid gets enlarged you get this large mass in your neck and that's a condition known as goiter now in the u.s you don't see much in terms of iodine deficiency you
don't see often people walking around with goiter think about if there's anything that you buy that is labeled that iodine is added to it so just think for a minute is there anything that you buy the iodine is added if you look at salt salt says iodized salt they add iodine to salt to sodium chloride to help protect against goiter because again all you need is a very small amount and it protects against disease and so that would be another example of a trace element needed in small amounts now remember i said that for an
element the element you cannot change the number of protons if you change the number of protons you change the element and the number of protons is referred to as the atom's atomic number so when we talk about atomic number we're talking about the number of protons so helium with two protons has an atomic number of what so what would helium's atomic number be if it has two protons answer it has an atomic number of two the mass number is the sum of the protons and the neutrons in the nucleus the number of electrons does not
factor into the mass number the reason for that is let me give you an analogy to explain the difference in mass between let's say a proton and an electron so imagine in one hand you have a bowling ball and in the other hand you have a lifesaver which one has a greater mass the bowling ball or the lifesaver and if you said the bowling ball you'd be correct right that bowling ball has a much heavier mass right think of in terms of weight weight and mass are not exactly the same but just to give you
an idea because we always think in terms of weight right the bowling ball has a much larger mass than the lifesaver that's like the difference between the protons and the electrons the protons mass is much much greater than that of the electron and so it makes the mass of the electron so small that it's negligible it doesn't matter because its mass is so small that it doesn't contribute to the overall mass so the bowling ball and the lifesaver are going to be very different so we don't need to include the mass of the lifesaver so
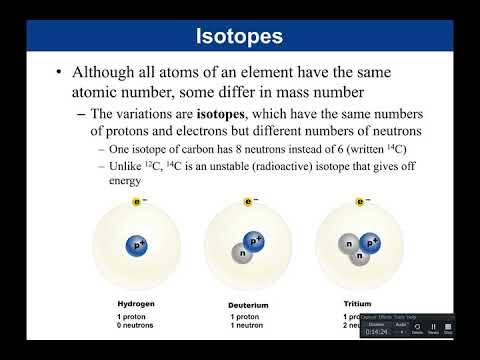
if we look at helium and we look in the nucleus of the atom you'll notice that we have two protons and two neutrons so what would helium's mass number be answer four helium has a mass number of four and it has an atomic number of two so mass number is going to be four atomic number is going to be two now although all atoms of an element have the same atomic number some differ in mass number so again you cannot change the number of protons or you change the element so if an element differs in
their mass number what that tells you is that the component of the atom that changes is not the proton but instead is the neutron and if the atom changes its number of neutrons we call it an isotope isotopes have the same number of protons and electrons but a different number of neutrons for example one isotope of carbon has eight neutrons instead of six so standard carbon has six neutrons but an isotope of carbon has eight and it's written c14 so the 14 is the superscript so c14 is an isotope of carbon normal carbon standard carbon
is carbon 12. this number again this 12 is the mass number number of protons plus number of neutrons carbon always has six protons so carbon 12 has six neutrons carbon 14 if the mass number is 14 and six of those are protons that means that carbon 14 has eight neutrons so what makes carbon 12 and carbon 14 different is that they have a different number of neutrons what makes them both carbon what makes them both carbon is that they have the same number of protons they both have six protons and that's why they are carbon
now unlike carbon 12 carbon 14 is unstable or radioactive and this radioactive isotope gives off energy it's unstable and it decays over time and so one of the advantages of having these radioactive isotopes is that in medicine they can be used as tracers because the cells can't differentiate between isotopes to a cell carbon is carbon whether it's carbon 12 or carbon-14 and so we can use these radioactive isotopes as a way to measure where an element is being used in the body for example if we were worried about a problem with the thyroid right the
thyroid has needs iodine so we can use radioactive iodine as a tracer because if you ingest if you take in that radioactive iodine the body doesn't know that it's radioactive and anywhere that iodine would be used that radioactive isotope would be utilized and those radioactive isotopes give off energy they're unstable and as a result we can measure them on machines and so we can see where these radioactive isotopes are being utilized by the body and so radioactive isotopes while not good in high doses right if you've ever seen about chernobyl for example radioactive isotopes are
not good but in very small amounts they can be useful for certain purposes like acting as tracers in medicine down below what you're looking at is hydrogen atom hydrogen atom has one proton and no neutrons what is hydrogen's atomic number hydrogen's atomic number is one what is hydrogen's mass number mass number is one because we have one proton and we have zero neutrons so it has a mass number of one notice it has one electron because in an uncharged element the number of protons equals the number of electrons so those are equal there's no net
charge deuteridium is an isotope of hydrogen it's still hydrogen because it has one proton and that one proton is characteristic of hydrogen however deuteridium also has one neutron one proton plus one neutron has a mass number of two deuteridium do refers to two so notice that my number of protons did not change i still have one proton i still have one electron but what is now different is going to be that neutron tritium tritium tri means three tritium has a mass number of three it's still an isotope of hydrogen because it has one proton but
it has two neutrons and so combined has a mass number of three and so that is isotopes so if we look at a periodic table you can see that these periodic tables are arranged in rows and columns so the top for example hydrogen and helium they're in the same row going down the periodic table those are referred to as columns and we'll talk a little bit more about the rows and columns in a little bit but what i want you to notice is that the number on the top of the periodic table that is an
atom's atomic number remember that the atomic number is equal to what and that is the number of protons the bottom number is referred to as the atomic mass that is going to be the number of protons just abbreviate number of protons plus number of neutrons right the atomic mass is the number of protons plus the number of neutrons now you might notice when you look at the periodic table that the numbers on the bottom are not always whole numbers so for helium it is but let's say we look at carbon for example notice that under
carbon it says 12.01 and you might wonder well how do you have .01 of a particle and the answer is you don't what that number is on the periodic table it's actually the weighted average of the isotopes meaning they weight that atomic mass based on how abundant that particular isotope is and so when you're trying to determine how many protons how many neutrons etc you just go ahead and round that number to the nearest whole number so let's look at an example so sodium here is sodium over here that's our sodium so what i want
you to do is i want you to pause and i want you to try and work through this on your own so for sodium based on that periodic table what is the atomic number what is the atomic mass based on that number how many protons will it have how many neutrons will it have and how many electrons will it have so pause your video and when you're ready when you work this out yourself push play and then go through and hear the answers so if i look at sodium the top number remember is the atomic
number so sodium has an atomic number of 11. if we look at the atomic mass that's the number on the bottom notice for sodium it says 12.99 so we're going to round that to 23. so if we look at sodium number of protons is going to be 11 because the atomic number is equal to the number of protons can that ever change answer is no it cannot be sodium and have a different number of protons so the number of protons does not change number of neutrons how many neutrons does sodium have answer 12 because the
atomic mass is the number of protons plus the number of neutrons atomic number is the number of protons so if we're trying to figure out the number of neutrons so number of neutrons is equal to atomic mass minus the atomic number that is going to give you the number of neutrons so that's why for sodium if we have an atomic mass of 23 and an atomic number of 11 23 minus 11 is going to give you 12 neutrons for sodium the number of electrons if the element is uncharged notice there's no charge next to sodium
the number of protons is equal to the number of electrons so this is going to be 11 because protons are positive neutrons are neutral and electrons carry a negative charge and so you should be able to look at a periodic table if given one on an exam and you should be able to tell me the atomic mass and the atomic number and then you should also know how many protons does that have how many neutrons how many electrons you do not need to memorize the periodic table if i ask you a question related to that
i will give you enough information to be able to answer that question so this is just showing you a different periodic table and i just kind of like to put this in here because it shows you some uses for some of these elements so like if we look at fluorine for example fluorine is used in toothpaste and it's used because it will inhibit bacterial growth so it's used to protect the health of your teeth lithium lithium so li over here lithium is used for example in batteries and so this is again just to kind of
show you some different uses for these different elements by no means do you need to memorize this table so when we talk about the structure of an atom again the nucleus of the atom is where we're going to find the protons and the neutrons around that nucleus is going to be the electron shell and so the electrons are going to exist in these distinct shells around the nucleus the first shell the one closest to the nucleus contains a maximum of two electrons that orbital only holds two electrons so if i look at hydrogen hydrogen has
an atomic number of one what that means is hydrogen has one proton because protons and neutrons cancel each other out that also means that hydrogen has one electron so it has one electron in its first shell helium helium has an atomic number of two which means that helium has two protons and two electrons so notice that its electron shells are going to contain two electrons if we look at lithium lithium has an atomic number of three what that means is that it has three protons what that also means is that it has three electrons two
electrons can occupy the first shell so notice that there's only two in the first shell which means that the third one right because there's going to be three electrons total the third one is going to exist in the second shell so the first shell the one closest to the nucleus holds a maximum of two electrons the second and the third shell both contain eight electrons maximum so like in neon where neon has eight electrons in its outer shell that outer shell is called the valence shell so the outermost shell is called the valence shell and
the outer electrons are referred to as the valence electrons it's the valence electrons that are going to dictate whether or not a chemical bond forms so i'll talk about that more in a minute so notice that neon has an atomic number of 10. what that means is that neon has 10 protons neon also then has 10 electrons two electrons in the first shell eight in the second shell right so two plus eight is going to give us our ten if we then look at sodium sodium has an atomic number of eleven what does that tell
us well that tells us that it has eleven protons in its nucleus total it also has 11 electrons 2 in the first shell 8 in the second so 2 plus 8 is 10 it has 1 in the third shell so the second and the third shell hold a maximum of eight that is what we call the octet rule oct refers to eight they hold a maximum of eight now there are exceptions to the rule there are times where um the second or the third shell could potentially have more than eight but for the sake of
this class and to keep it simple we're gonna go with what usually happens and that is that the second shell holds a maximum of eight and the third shell holds a maximum of eight as well you won't need to understand those um exceptions to the rule so this is a question for you oxygen is an element with an atomic number of eight based on this information which of the following statements is true red oxygen can be broken down into simpler component substances yellow each oxygen atom will always have eight protons green each oxygen atom will
always have eight neutrons blue each oxygen atom will always have four electrons or purple protons plus electrons is eight so for this question i'm not going to provide the answer in the video instead what i'm gonna do with this question is i'm going to put on canvas a practice quiz and the purpose of the practice quiz is for you to familiarize yourself with the protocol needed for respondus which is our online testing protocol so that you can test in this practice quiz without it being your actual exam that way you can make sure that respondus
is working properly this will be graded simply for participation meaning that as long as you do the practice quiz you will get your points but again the practice quiz is more for you just to make sure that your computer setup is ready to work with respondus so this will have a big window for you to do it um but you definitely want to do this before you have any of your tests so that way you can make sure that you can use respondus so i will post the answer on the quiz you'll get feedback right
away when you take the quiz to make sure that you get these answers correct so we won't cover them in the video so now we're going to look at chemical bonding electron arrangement determines the chemical property of an atom only electrons are involved in chemical activity chemical bonding comes about when atoms seek their lowest energy state when they are the most stable an atom achieves a state when it has filled its outer electron shell so atoms will form chemical bonds in order to fill its outer shell so to accomplish this to fill their outer shell
atoms can share donate or receive electrons so there's multiple ways that they can fill their outer shells this results in attraction between atoms which we then call a chemical bond so if two atoms are sharing electrons that bond between those atoms those are going to be your chemical bonds or if one atom donates and one receives those two atoms are going to be attracted that's going to form a chemical bond and so again atoms are going to seek out and try and fill their outermost shell because that's when they are most stable so there are
three types main types of chemical bonds we have a covalent bond covalent bond is where atoms share one or more electron pairs we have an ionic bond where one atom is going to lose and another atom is going to accept electrons so one is going to donate one is going to receive and then those elements are going to be attracted to one another and then the last one is going to be the hydrogen bond and the hydrogen bond is where we have a covalently linked hydrogen so a hydrogen that's already in a covalent bond that
can react with an electronegative atom like oxygen i will come back to this definition a little bit later on in this lecture because you have to understand things like electronegativity in order for a hydrogen bond to make sense but i just wanted to put it here because this slide just summarizes our three types of chemical bonds so if we look at our periodic table so notice that our rows are basically aligned based on how many shells they have so notice that the first row the top row only has electrons in the first shell the second
row means that those atoms have electrons in the second shell the third row has electrons in the third shell so now if we look at the columns right going down notice that we have hydrogen lithium and sodium all three of those atoms have one valence electron so i want you to look at what do the elements on the far right of the periodic table have in common so what does helium neon and argon all have in common so those three elements what they have in common is that they all have their outer shells full their
outermost shell is full because helium has two it only has two electrons in that shell neon argon have eight in the second and third shell respectively those outer shells are full so these particular elements are unreactive they do not need to form chemical bonds because their outermost shell is already full remember that the reason that atoms form chemical bonds is to fill their outer shell those elements don't need to form chemical bonds because their outermost shell is full and they're already stable so that's what those columns in the periodic table are for so this is
just showing you some electron configurations of different atoms and so you'll notice that this is the electron arrangement for the main four remember that the main four elements are hydrogen carbon nitrogen and oxygen those make up 96 of the human body when we talk about our macromolecules in the next part what you're going to see is that these four elements are the main components in macromolecules so these have a very important role hydrogen has an atomic number of one what that means hydrogen has one proton so it has one proton in its nucleus it has
one electron in its first shell and it needs one more to fill its outer shell so it has one it needs one so notice that this element is half full carbon carbon has an atomic number of six what that means is that carbon has six protons what that also means is that carbon has six electrons two in the first shell four in the second shell because two plus four is six so in its second shell carbon has four valence electrons and it needs four more carbon is also half full you're going to see that later
on the fact that carbon and hydrogen are both half full is an important concept and i'll come back to that later nitrogen has an atomic number of seven atomic number of seven means seven protons it also means that nitrogen has seven electrons two electrons in the first shell five in the second shell two and five is seven notice that nitrogen needs three more one two three oxygen oxygen has an atomic number of eight atomic number of eight means that oxygen has eight protons it also means that oxygen has eight electrons two electrons in the first
shell six in the second shell two and six right will make eight oxygen needs two more now the reason that i'm pointing this out is the easiest way to remember how many electrons these need is i remember the acronym honk except that the end is a c and not a k so honk hydrogen is going to form one bond meaning it only needs one more electron to fill its outer shell oxygen needs two right it needs two more valence electrons nitrogen needs three carbon needs four so hydrogen needs one to fill its outer shell oxygen
needs two nitrogen needs three carbon needs four so you can remember that as honk these four elements if i ask you questions related to these four i will not give you atomic numbers you should know these four they're not hard because again if you can remember honk one two three four that's the number of electrons more that they need so this is just showing you some different electron configurations so this is a class demo i will do this class demo on the zoom so just be aware that we will cover this on the zoom meeting
a compound is a substance consisting of two or more different elements key here being different so two or more different elements combined in a fixed ratio and there are many compounds that consist of only two elements an example would be table salt or sodium chloride which is also written nacl the na is for the sodium the cl is for the chloride and interestingly sodium itself is a metal and chlorine is a poisonous gas yet when you put this metal and this poisonous gas together you get this emergent property and this emergent property is that now
by mixing these two elements you get a new compound which is sodium chloride and sodium chloride when combined is an edible compound and so this is an example of a compound and an emergent property so now we're going to move on and talk about the different types of chemical bonds so we'll start with the covalent bond and a covalent bond forms when two atoms share notice share one or more pairs of electrons and a molecule is formed when two or more atoms are held together by a covalent bond the molecule could be two of the
same elements they could be different elements that part doesn't matter but the atoms are held together by a covalent bond so an example of a molecule would be water h2o and h2o means that there are two hydrogens and one oxygen if we look at oxygen for example oxygen has an atomic number of eight what that means is that oxygen has eight protons that also means that oxygen has eight electrons we have two in the first shell and we have six in the second shell so if oxygen has six in its second shell how many more
electrons does it need to fill its outer shell and the answer is it needs two so what happens is we have two hydrogen atoms hydrogens have one proton and one electron and in order to fill both of their outer shells because remember that chemical bonds form in order to fill outer shells so to fill both of their outer shells oxygen and hydrogen are going to share a pair of electrons now we can color code this so the one from the oxygen will make be red and the ones from the hydrogen will be blue so that
when they come together and they share each going to be sharing one that came from hydrogen and one that came from the oxygen and so they're sharing that pair of electrons and so oxygen is participating with one of those electrons hydrogen is participating with the other but by sharing notice that we've now filled both of their outer shells because now oxygen has starting up here one two three four five six seven eight so oxygen has eight in its second shell hydrogen has two in its first shell both of these so now we filled both of
their outer shells so again chemical bonds are when atoms are going to seek to fill their outer shells and they do that by forming these chemical bonds and in the case of the covalent bond they're going to share a pair of electrons and by sharing it fills both atoms outer shell and so this would be a covalent bond so here are some different examples of a covalent bond so we have hydrogen h2 hydrogen is a gas and h2 notice there are two hydrogens together it's still a molecule because they're held together by a covalent bond
notice that if you look in the electron diagrams you can see that they are sharing one pair you can see this in the lewis dot formula so this is the lewis dot structure where we draw the electrons as dots so they're sharing one pair you can also see a single covalent bond written as one line one line represents a single covalent bond meaning that those elements are sharing one pair of electrons you can also see this distributed as a space filling model where you can see that there's two hydrogens together and in this case they're
held together again by a covalent bond if we look at oxygen o2 oxygen remember as a gas this is the gas that we breathe in oxygen needs two more electrons to fill its outer shell so for oxygen they can't just share one pair of electrons if they share one pair each oxygen only has seven so instead what happens if you look in the lewis dot structure they are sharing two pairs of electrons and by sharing two pairs now each oxygen has eight and has filled its outer shell notice that this is represented by two lines
when you see two lines between two atoms that tells you that that is a double bond there are triple bonds typically that's with carbon meaning that they're sharing three pairs of electrons because remember that carbon forms a maximum of four bonds water h2o again they're going to be sharing one pair of electrons so we have two hydrogens and one oxygen we could have methane ch4 the carbon is sharing with the hydrogen so notice that it's four single bonds because again carbon needs to form four bonds to fill its outer shell and so these are just
some examples of different covalent bonds to understand about types of covalent bonds we need to understand a little bit about electronegativity and electronegativity notice electronegativity is the affinity that an atom has for electrons electrons have a negative charge so a general kind of rule of thumb is that atoms that we say have a high electronegativity means that they have a high attraction or a high affinity for electrons they really want electrons to fill their outer shell and that's because these are going to be atoms where the outer shells are nearly full they might only need
one electron or two electrons to fill their outer shell and so because they only need a few electrons to fill their outer shell they have a high affinity for electrons they really want that last electron or those last two electrons to fill their outer shell an example of an atom that is highly electronegative would be chlorine chlorine has an atomic number of 17. what that means is that chlorine has 17 protons if chlorine has 17 protons how many electrons does it have 17. how many in the first shell 2 how many in the second eight
right two and eight brings us to ten which means that it has seven in its third shell so notice starting up here one two three four five six seven how many more electrons does chlorine need to fill its outer shell it only needs one so because chlorine only needs one electron to fill its outer shell it is highly electronegative highly electronegative it has a high affinity for electrons because it really wants that last electron to fill its outer shell so atoms that have outer shells that are nearly full are highly electronegative so chlorine for example
oxygen because oxygen needs two nitrogen because nitrogen needs three etc atoms with very little electronegativity have a low attraction for electron they have a low affinity for electrons and in that case the outer shells are nearly empty so let's look at an example so let's say we're looking at sodium sodium has an atomic number of 11. how many protons 11 how many electrons 11 2 in the first shell eight in the second that brings us to ten one in the third shell so how many electrons would sodium need to acquire to fill their outer shell
how many would it need to take in to fill its outer shell answer is it would need to gain seven more is it likely that sodium is going to be able to pull seven electrons from somewhere else no right not very likely so in the case of sodium it's not trying to pull electrons it's not trying to have an affinity for electrons it doesn't want them instead it would rather give them away because if sodium gives away this one valence electron that second shell is full and now it's stable so atoms like sodium have very
little electronegativity they don't have the affinity for the electrons instead they would rather give them away and so atoms that have very little electronegativity have outer shells that are nearly empty so this would include hydrogen carbon sodium these are all not very electronegative now sometimes students get a little bit confused about hydrogen because if you recall hydrogen has one valence electron in the first shell and it only needs one more so students often go well it only needs one more yes but if you think about big picture if they only have two electrons in the
first shell and they have one out of two that outer shell is only half full if you think about carbon carbon has four electrons in its outer shell it needs four more carbon is also half full it has half of its valence electrons and so as a result hydrogen and carbon actually have very similar electronegativities because they're both half full they both have half of their valence electrons and so they're not very electronegative it's going to be hard for them to fill those outer shells so if we look at the periodic table and we look
at the electronegativities again the far right column on your periodic table helium neon argon these are going to be elements that are unreactive because their outer shells are already full fluorine chlorine these are electronegative they both have seven valence electrons they only need one more oxygen and sulfur need two more they're electronegative but a little bit less than the atoms to the right so fluorine is more electronegative than oxygen chlorine is more electronegative than sulfur that's why you're seeing this electronegativity go down nitrogen and phosphorus less electronegative than the ones to the right and then
once we get here where we're at carbon and silicon which are both half full this diagram is a little misleading because it's showing like it's still going down but in fact it's more like this where all of these elements from half full and less these all have very little electronegativity they don't have a high affinity for electrons because they would need to acquire too many so these elements at the halfway mark the carbon and silicon and everything to the left of that those atoms have very little electronegativity they don't have a high affinity for electrons
because that it would be too difficult for them to acquire the electrons that they needed they would rather maybe give away electrons for example so if we talk about a covalent bond where the atoms are sharing electrons we can talk about what are called polar covalent bond and nonpolar so we'll start with our polar covalent bond polar covalent bond is when we get unequal sharing of electrons and unequal sharing of electrons means that they're not sharing equally think of it like a tug of war right so if you have you know a 300 pound heavy
duty like lifter and you have this kid who weighs 50 pounds right and they're doing a tug of war who's gonna win the 300 pound weight lifter or the 50 pound kid the 300 pound weight lifter right same idea when you're sharing electrons if atoms have different electronegativities if one is much more electronegative than the other when they share electrons right they're sharing it's between the two they're not sharing equally the one with the greater electronegativity the one that has the higher affinity for the electrons is going to pull harder for those electrons and as
a result those electrons are going to spend more time around the atom that is electronegative so if we look at water for example h2o oxygen and hydrogen which one is more electronegative oxygen it has six valence electrons and therefore it only needs two more remember we said that hydrogen is only half full it has very little electronegativity so when oxygen and hydrogen share they will share but they won't share equally oxygen has a greater pull for the electrons it has that greater affinity so what ends up happening is is that the electron spends more time
around the oxygen than it does the hydrogen electrons are negatively charged so what you get is you get this partial negative this partial negative charge because that electron is not fully being stolen by oxygen oxygen isn't taking the electron but the electron is spending more time around the oxygen and therefore the oxygen gets that partial negative charge hydrogen then as a result gets a partial positive it gets a partial positive because remember that a hydrogen atom is simply a proton and an electron right so this hydrogen has one proton and if its electron is spending
more time with oxygen and the electron is spending more time with oxygen that means that the hydrogen is left with more of a positive charge because it has the proton but the electron is not spending as much time so the hydrogens end up with the partial positives and the oxygen ends up with this partial negative this ends up with what we call a polar molecule this molecule is polar it has this charge associated with it it's not a full charge but it is a partial charge and that will also affect the way that molecules interact
because if you have these charges on this molecule they can interact with other things that are charged charged as well so for me the way that i remember polar i think polar pulling if you're talking about a polar covalent bond one atom is pulling harder than the other and that's going to cause unequal sharing of electrons so if you're asked a question that asks you to identify the type of chemical bond first thing you want to look at is you look at the atoms and you see do they have similar electronegativities or different in the
case of oxygen and hydrogen they have different electronegativities and as a result because they're not having the same affinity for electrons when they share they're not going to share equally the electrons are going to spend more time around the electronegative atom than they will the atom that is not very electronegative so if we look at the main four elements in terms of their electronegativity oxygen is the most electronegative because it only needs two followed by nitrogen so nitrogen is a little bit less electronegative because it needs three and notice carbon and hydrogen are equal they're
roughly the same they're less than nitrogen and oxygen but they're roughly the same because they are both half full so if you're talking about oxygen and hydrogen that's going to be polar right because oxygen and hydrogen have similar or i'm sorry they have different electronegativities and so that's what you're looking for if you're trying to see if a bond is going to be a polar covalent or a nonpolar covalent so a nonpolar covalent bond nonpolar non-pulling and what that means is that electrons are shared equally so when these atoms form a chemical bond and they're
sharing electrons neither one has a greater affinity for the electrons neither one is pulling harder so this is going to occur between atoms with very similar electronegativity and because they have a similar electronegativity neither one has that greater affinity for the electrons so the electrons are going to be shared equally if the electrons are shared equally no partial charges are created because the partial charge comes from when the electron spends more time with one atom compared to another but in this case if they're shared equally neither one is going to get a partial negative or
a partial positive the molecule itself is going to remain uncharged it's going to have no net charge no overall charge on the molecule because the electrons are being shared equally what types of molecules will be nonpolar hydrocarbons hydrocarbons refer to the fact that they are primarily carbon and hydrogen so like lipids for example if i look at a fatty acid notice it has this long hydrocarbon chain that long hydrocarbon chain carbon and hydrogen remember have similar electronegativities so when they share they will share but they will share equally there's no charge on this molecule and
so again this is going to happen when you're talking about elements or atoms that have similar electronegativities if they are similar they are going to share their electrons equally so now we're going to talk about the ionic bond so we just finished with the covalent bond where we were talking about sharing electrons now let's look at the ionic bond where one atom is going to donate electrons and the other is going to receive them so ionic bonds form when electronegativities differ too much meaning that one atom is so much more electronegative than the other that
it's simply going to steal the electrons from the other atom so if we look at sodium sodium again has an atomic number of 11. so what that means is that sodium has 11 protons right it has 11 protons in an uncharged sodium atom if it has 11 protons it also has 11 electrons so that it has no net charge so notice that if we look at the diagram of sodium it has two electrons in the first shell it has eight in the second so two plus eight is ten it has one electron in the third
shell let's look at chlorine chlorine has an atomic number of seventeen what that means is that for protons they're equal to 17. in terms of electrons if we're talking about an uncharged chlorine the number of electrons is also 17. so we get no net charge so if we have 17 electrons in the first shell we have two second shell we have eight so two and eight is 10 which means that there are 7 in the third shell because there's 17 total so there's 7 in that third shell so if we have 7 in that third
shell chlorine only needs one more to fill its outer shell sodium on the other hand would have to acquire seven more to fill its outer shell so do sodium and chlorine have similar electronegativities answer is no they are very very different chlorine is very electronegative sodium has very little electronegativity because it's not going to be able to acquire 7. what does sodium do instead it gives away its lone valence electron and when it gives it away now you're going to end up with what's called an ion an ion is an atom that has a different
number of electrons and as a result ends up with a charge now first let's talk about why why would they form an ionic bond versus a covalent bond if sodium and chlorine were to share a pair of electrons right if this pair here were to be shared that pair of electrons if they were sharing does that fill both of their outer shells answer is no it does not fill their outer shells it would fill chlorine's outer shell but it's not going to fulfill sodium's outer shell because that would still only have two electrons in sodium's
third shell it needs eight to fill its outer shell so sharing that pair of electrons going to happen sodium is going to give it to chlorine so now if i look at my sodium ion how many protons does my sodium ion have sodium ion still has to have 11 protons because remember if we're calling it sodium can the number of protons change answer is no cannot change the atomic number is still 7 and as a result the number of protons is still 11. that cannot change if you change the number of protons you change the
element but how many electrons does sodium now have so notice that when it gave away that third shell when it gave away that one electron in its third shell it has two in the first shell and it has eight in the second shell so two and eight is ten so now sodium has 11 positive charges and 10 negative charges so what is the overall net charge on sodium plus one it has one more proton relative to the electron so sodium ends up with this positive charge there is a particular name for ions that have either
a positive or a negative the positively charged ion we call the cation the way i remember this cation has a t in it it has a plus in it the cation is the positively charged atom it's the one that's going to have the positive charge so sodium becomes a cation becomes positively charged if we look at chlorine chlorine still has 17 protons because again we cannot change the number of protons and have it still be chlorine so it has 17 protons and how many electrons does it now have well if it gained one electron it's
no longer 17 it's now 18 electrons notice that fills chlorine's outer shell it now becomes a chloride ion the chloride ion has eight valence electrons now so the overall charge on this ion notice if we compare negatives and positives we get a negative one charge right we have one more electron relative to protons so this ion becomes negatively charged that is what we call the anion n negative so i remember the cation the t is the plus that's the positively charged ion the anion is the negatively charged the n in the middle tells you negative
so now we have a sodium ion that is positively charged we have a chloride ion that is negatively charged and if you think about charges if you know anything about playing with magnets and you know anything about charges do like charges attract or do opposites attract answer is opposites attract so the paula abdul song is right opposites do attract for atoms so this positively charged sodium is going to be attracted to that negatively charged chloride ion these opposites attract and same thing if you're playing with magnets if you've ever played with magnets you'll know that
when you turn them one way they will attract and the magnets will come together but if you try and turn one the other way and then stick them together they don't go together they repel like charges repel opposites though will attract and so let's see why that's important so when we have those oppositely charged ions because one atom gave electrons one donated and the other received what you're going to end up with is your ionic bond so think of ionic bond between ions oppositely charged ions so in this little crystal here the yellow represents the
sodium and the green represents the chloride what you get is you get this crystal lattice where you get these alternating sodiums and chlorides and sodiums and chlorides because the positives are attracted to the negatives so you end up with this salt crystal and so now you get this new emergent property because now both of those elements are stable once they form that chemical bond they both fill their outer shell and we get our emergent property we now get table salt which is edible and so ionic bond is when one atom donates the other receives you
end up with oppositely charged ions they attract and when they attract they're going to come together and form that ionic bond so this will be another question on your practice quiz so oxygen and hydrogen differ and their electronegativity thus red they can share electrons but unequally yellow sometimes the negative charge on the electrons turns into a positive green they can share electrons equally blue hydrogen is attracted to oxygen but does not bond with it purple they have the same number of protons so again i will post this on the participation assignment as a practice quiz
just to make sure that your respondus is working properly and i will also provide the answer there as well so now we're going to move on and talk about the hydrogen bond and remember that this is that definition that i said that you probably wouldn't understand that definition until we talked about electronegativity so a hydrogen bond is a weak attraction between a covalently linked slightly positive hydrogen atom one that's already bound to oxygen or nitrogen and that already covalently linked hydrogen is attracted to a slightly negative atom like oxygen or nitrogen so what does that
mean well if you think about water remember that water is polar and oxygen has that partial negative right so here's the partial negative hydrogen has the partial positives so what we end up with is we end up with this molecule that has these opposite charges so if you think about this and you think about the electrons let me change the ink to black if you think about where hydrogen is going to have its electrons oxygen and hydrogen have their electrons so remember that total within a water molecule the oxygen is going to have eight there's
a pair shared with hydrogen there's a pair shared with hydrogen and then there are two what we call lone pairs so this is one pair here's another pair the oxygen is partially negative and the hydrogen is partially positive so notice that we have an already covalently linked hydrogen there's my hydrogen that's already in a covalent bond and it is interacting with an electronegative atom like the oxygen so it has this weak hydrogen bond those opposite charges are attracted this bond is not very strong so if you think about like a covalent bond where they're actually
sharing that bond is relatively strong in terms of a hydrogen bond we're talking about an attraction between opposite charges that interaction is much weaker so that's going to be an already covalently linked hydrogen to an electronegative atom like oxygen so in this case you're seeing that for each water molecule each water molecule can participate in a maximum of four hydrogen bonds we can have one per hydrogen and then one per lone pair of electrons so four hydrogen bonds can form at a maximum per water molecule so when you think about a hydrogen bond what you
want to think about is that this is between molecules the hydrogen bond is going to link molecules together it's not within the same molecule this right here that bond between the oxygen and the hydrogen that is not the hydrogen bond remember that that is the covalent bond the the hydrogen bond is the one that's between the molecules not within one molecule but it's between molecules it's holding molecules together and so that's the hydra that's a hydrogen bond an already covalently linked hydrogen to an electronegative atom like oxygen if you go back to the slide before
you can see that we can see a hydrogen bond not just between water but water and ammonia can also form a hydrogen bond and that's because the water molecule the hydrogens are partially positive they can be attracted to the partially negative nitrogen because nitrogen is more electronegative than hydrogen so they don't share equally so hydrogen bonds can occur between an already covalently linked hydrogen to an electronegative atom like oxygen or nitrogen so again this will take place in the zoom session that we're going to talk about so in our zoom we will answer this question
and you guys will discuss it with other members of the class you have nitrogen with an atomic number of seven and hydrogen with an atomic number of one what type of bond will form between hydrogen and nitrogen and how many hydrogens will bond with each nitrogen so you can start working on this ahead of time and then we will also go over this in the zoom session so now we need to talk about chemical reactions so chemical reactions involve the making or breaking of bonds between atoms a change in chemical energy occurs during a chemical
reaction if we call a reaction endergonic endergonic think of energy has to put in so these reactions absorb energy energy must be put in so i remember endergonic in you have to put in energy exergonic reactions release energy energy exits meaning that it's being released so an endergonic reaction is one where you have to put in energy an exergonic reaction is one where energy is released so let's look at some examples so if we're going to form a bond if we're going to put atom a and atom b together or ion a and i on
b if i'm going to build something right if i'm going to build something compare building something to breaking something down right let's say i'm trying to build a tower i have blocks and i'm trying to build a tower is it gonna take more energy being put in to build a tower or to break it down which one's going to require a greater investment of energy the answer is building right because if i'm building something it's going to require more energy than it would be if i was breaking it down so when we have these synthesis
reactions where we're building something where we're forming new bonds and we're making molecules bigger that is going to be an endergonic reaction endergonic because energy has to be put in you have to put in energy to build that larger molecule so for example if your cell is going to store sugar the way that animal cells store sugar is going to be through a molecule called glycogen glycogen is your storage sugar so if you form glycogen you have to link glucose molecules together you have to put glucose together to form this bigger polysaccharide this bigger sugar
that's an endergonic reaction energy has to be put in so this is going to be endergonic we have to put in energy to form this molecule anabolism is the synthesis of molecules in the cell so the way i remember this one notice the beginning if you think of anabolic steroids if you've ever heard of anabolic steroids why does somebody take anabolic steroids what are they trying to accomplish the answer is they're trying to build muscle right so anabolism is referring to the synthesis or the building of molecules in the cell and those types of reactions
are going to be endergonic energy has to be put in for that new molecule to be built on the flip side if we have what we call a decomposition reaction if we're breaking something down so if we're taking a b and we're breaking it apart into a plus b if we're breaking something down we're breaking that bond we are going to release the energy that's stored in those in that bond so the bond between a and b has energy stored when we break that bond we're going to release energy and that reaction is going to
be exergonic energy is being released catabolism is the decomposition reaction in the cell meaning the breakdown of molecules the way that i think of catabolism i think it's a catastrophe it's like it all falls apart it's coming apart it's being broken down so catabolism is the decomposition reaction it's the breakdown of bigger molecules into the smaller parts and when we break those bonds we are going to release the energy that is stored in that bond and we will learn a lot more about this when we get to the metabolism chapters some reactions are what we
call an exchange reactions where we have a synthesis and a decomposition coupled together an example of this is if we have naoh which is sodium hydroxide and hcl which is hydrochloric acid if we mix an acid and a base together you might know that you get what we call a neutralized solution they will cancel each other out and the reason for that is is that we are going to break these apart so this is going to break apart those two pieces this is going to break apart the hydroxide this oh is going to form a
bond with the hydrogen and the sodium is going to form a bond with the chloride and so we've rearranged it and the products that we get are sodium chloride so nacl plus our water molecule so this is an exchange reaction all we're doing is simply swapping pieces and so that would be an exchange reaction some reactions can be what we call reversible meaning that under certain conditions the reaction will go one way and in another the reaction will go in the other direction so each direction might need a special condition to allow that to occur
so one of the things that i have here and i switched these originally they were on the slide backwards the heat was at the top and the water was at the bottom but in fact it should be the water this way if we break down a and b if we're breaking down a and b you're going to see this when we get to macromolecules but breaking down of macromolecules is what we call a hydrolysis reaction hydro is referring to water lysis is breaking so we use water to break it down so this is showing that
if the reaction is going this way it requires water so if we want to break down we want to hydrolyze a and b we have to put in water and then we're going to break it down to a plus b however if we have a plus b and now the condition is heat heat is energy it's kinetic energy it's the energy of motion so if we apply heat the energy from heat can be used to form a and b to go together because remember if we're building something energy has to be put in so putting
a and b together heat might facilitate that reaction it might make that reaction possible so that now you build a and b so this is what we call a reversible reaction under certain conditions it goes one way and under a different set of conditions it goes the other way not all reactions are reversible but some are now when we talk about organic compounds organic compounds always contain carbon and hydrogen carbon and hydrogen so if you hear of the field of chemistry that's organic chemistry organic chemistry is dealing with molecules that involve carbon and hydrogen so
our macromolecules for example would be in organic compounds so organic compounds contain carbon and hydrogen inorganic carbon or inorganic compounds typically lack carbon or have carbon but not hydrogen so an example of this would be like carbon dioxide co2 co2 would be an inorganic carbon yes it has carbon but it doesn't have carbon and hydrogen so that's why this would be an inorganic compound so inorganic compounds typically lack carbon or they may have carbon but not hydrogen also so they don't have carbon and hydrogen if they have carbon and hydrogen they would be called an
organic compound inorganic means that they don't have carbon and hydrogen together so we're going to move on now and talk about important properties of water why is water so essential to life on earth so the cell is primarily water anywhere from 70 to 95 of the cell is going to be water and the rest are going to be carbon-based compounds primarily so our sugars our proteins etc so what this tells you is that water must be doing something very important because why else is the cell 70 to 95 percent water water must be doing something
important and so we're going to look at what are the properties of water that make it essential to life on earth so just a reminder of water structure water is both a compound and a molecule it's a compound because it's two different elements it's hydrogen and oxygen it's a molecule because it is covalently bound right so two hydrogens are covalently bound to an oxygen that's where this notation comes from h2o the h2 refers to two hydrogens the o refers to just one oxygen so if we look at h2o remember that this molecule is going to
be polar because oxygen and hydrogen have different electronegativities so when they share electrons they do not share equally oxygen is more electronegative oxygen becomes partially negative the hydrogen as a result becomes partially positive so water is a molecule that is polar so what you're going to see is that the important properties of water are because water is polar and because water forms hydrogen bonds and so we'll walk through that so the first property that makes water so important for life is that water has cohesion cohesion is the tendency of molecules of the same kind to
stick together so water molecules display cohesion they're going to stick together and that's because they can hydrogen bond with one another and we saw that right that each water molecule can form a maximum of four hydrogen bonds this again is between water molecules so it's not within one water molecule but between water molecules this is a weak bond so the bonds these hydrogen bonds are constantly changing they're breaking reforming breaking reforming and so this is in in motion now why is cohesion important one reason that cohesion is important is that it accounts for water transport
in plants it's a process that's called transpiration transpiration sorry for the messy writing so transpiration if we think about plants plants remember are autotrophs they are self-feeders they can do photosynthesis they're able to make their own food and the way that they can make their own food is that they can take carbon dioxide water and sunlight and they can build their own sugars now where does photosynthesis take place in the plant well photosynthesis takes place in the leaves the leaves are where the chloroplasts are that's the organelle where photosynthesis is going to take place so
photosynthesis is going to happen in the leaves now one thing you'll learn later on is that the leaves on the bottom side of the leaves are little pores that are called stomata so the stomata are the pores on the underside of the leaf when those pores are open right when those pores are open the reason they open is that it allows the co2 gas in because remember that you need co2 the carbon dioxide to do photosynthesis so the reason that the pores open is to get in carbon dioxide the other thing that happens when those
pores are open is if you think of evaporation right evaporation is the loss of water transpiration means that water molecules come out of those pores they evaporate out of the pores now water has cohesion it has the ability to stick together so as this water molecule leaves its hydrogen bound to the next one which is hydrogen bound to the next one it's like it's pulling a chain of water up the leaves up to the leaves if you think about where you water a plant right if you're trying to grow a plant do we water the
leaves answer is no we water the roots the roots are the part of the plant that are used for water absorption they have these hairs which help increase surface area to increase to increase water absorption so the water goes in through the roots but it needs to get up to the leaves where photosynthesis takes place do things naturally want to go up if i were to hold a pen and let go is it spontaneously going to go up answer is no right gravity pulls things down so what that tells you is something is pulling the
water up the plant what is that something that's our transpiration when those pores are open and that water molecule leaves because water has cohesion and those molecules stick together that's going to pull the water up the plant it's going to pull it up from the bottom from the roots up to the leaves where photosynthesis can take place so water having cohesion is really important for the transport of water up a plant because you need to have water molecules stick together to pull the water up um the plant because it's going against gravity the other reason
that cohesion is important is that it accounts for surface tension if you've ever taken a glass and you've tried to fill it with water you might notice that you can fill it a little bit above the top of the glass that's because those water molecules stick together it has this surface tension which keeps it from going over the glass now eventually if you keep filling it up it will go over but it will allow you to fill it a little bit above the top surface tension is also responsible for these water striders these water walking
insects that can walk across water and that's because that water has enough surface tension that the insect can walk along it cohesion also is important for drops of water like if i were to take wax paper and i were to put water on the wax paper well the water is going to stick together up to a point and that's because water has cohesion it sticks together water also has a great capacity to absorb and retain heat this has to do with a quality called specific heat specific heat is the amount of energy required to raise
the temperature of a substance by one degree celsius water has a high specific heat and what that means is that it requires a greater input of energy to get the temperature to go up by one degree temperature is basically a um a measurement of the rate of vibrations of the molecules so when you apply heat to a liquid what happens to molecular motion what happens to the movement of those molecules well if you apply heat molecules move faster right those molecules start bouncing around they're going to start to vibrate and when they vibrate that's going
to increase temperature right so adding heat is going to cause molecular motion to increase which is going to then raise the temperature which is a measurement of the rate of the vibrations of the molecules however when heat is applied to water remember that water molecules have cohesion they stick together that hydrogen bond between water molecules is going to restrain some of the bouncing meaning that at any given amount of heat the temperature rises more slowly with water per unit heat so water at any given temperature has more heat than most liquids it stores that heat
because it has you have to input more heat to raise the temperature by one degree because again temperature is a measurement of the rate of movement of the molecules because water can hydrogen bond right because it has cohesion and it sticks together that's going to restrain that bouncing so they're not going to bounce as quickly and therefore more heat has to be applied to get the temperature to go up by one degree so if you've ever been cooking and you heat up oil or you heat up water which one is going to heat up faster
answer is the oil is going to heat up faster and that's because again water has a high specific heat it takes a lot more input of energy to get the temperature to raise by one degree celsius and that's because water has hydrogen bonding which restrains some of that bouncing the reason that this is important for life on earth is that this property that water has a high specific heat accounts for moderate climates around bodies of water and that's because water absorbs and stores heat in the summer with little temperature change and then it releases heat
in the winter so think about us in california what we call winter most of our country would say that's not winter our winter is not their winter but to those of us that live in california we think of you know in the 60s and i'm wearing boots and a sweater because i think that's cold that's because we've grown up in an area where we have the large pacific ocean next to us and it's able to moderate the temperature and to keep our temperature relatively constant so we don't get these huge fluctuations in temperature that other
parts of the country get when they actually get you know minus 10 degree weather and it's snowing and it's a blizzard we don't really see that next to the ocean right we don't really have that type of temperature because we have that large pacific ocean which helps moderate our temperature and helps keep our environment at a relatively constant temperature the other property about water is that ice is less dense than water so what does that mean if we say that ice is less dense than water well first we have to remember that temperature right has
to do with molecular motion so if we're comparing liquid water and we're comparing ice ice is a colder temperature liquid water is at a warmer temperature which one is going to have more molecular motion the ice or the liquid water answer is going to be the liquid water because liquid water has a higher temperature therefore the molecules are moving more and what that means is that if the water molecules are moving those hydrogen bonds are constantly breaking and reforming and breaking and reforming and so at any one time on average a water molecule is only
involved in about 3.4 hydrogen bonds it's not forming its maximum four because these molecules in liquid water are in motion those bonds are breaking reforming breaking and reforming in ice when the temperature goes down right when temperature decreases molecular motion decreases and as molecules start to slow down or they stop moving if the temperature gets cold enough that means that now with the water not moving the water molecules are going to be able to form their maximum of four hydrogen bonds and what happens is when they form their maximum four hydrogen bonds they form this
crystal lattice structure and what ends up happening is if you look at the ice and you look at density which is the amount of water molecules per space per given area the ice is less dense than the water notice that in the ice the water molecules are much more spread out than they are in liquid water so ice is less dense than water you've seen this if you ever put ice in a cup and you drink ice water right when you add the water the ice is going to float to the top because the ice
is less dense than water so why does that matter well because if you think about large bodies of water and when they freeze in the winter the top of the water is going to freeze but the ice is less dense than the water so the ice is going to remain at the top but the bottom is going to remain liquid water and so this is essential for life on earth because lakes and oceans don't freeze solid the top is going to form ice but the bottom is not and you've probably seen this in movies when
people fall through the ice right that's because the top is ice but underneath is liquid water and that's an important property because that allows life to exist underneath where the ice is formed so ice is going to expand right when frozen it's gonna spread out and it becomes less dense you've probably seen that water expands when frozen if you've ever made the mistake of putting let's say a soda can in the freezer like you go to the grocery store and you buy soda and you want to drink it soon so you take that soda and
you put it in the freezer to try and cool it down very quickly well if you're like me and you get distracted and you forget that you stuck that in there you might come back to open your freezer to find a very big mess because if you leave that soda in there and the water in there expands when frozen it's going to expand within the aluminum and it's going to cause that soda to explode and that's because water expands when frozen that's different than a lot of other things things like metal for example tend to
constrict when frozen but ice has this unique property in that it actually expands when frozen and so when ice expands when frozen it's now less dense than liquid water and so that allows life to to live under the ice another important property is that water is a solvent for life and what is a solvent well a solvent is a substance which something is being dissolved so the solvent is what's doing the dissolving the solute is what's being dissolved so if i take salt and i mix it with water right my salt is what's going to
be dissolved this is my solute and my water is going to be my solvent it's what's doing the dissolving so i mix my salt in my water i maybe stir to mix it and what i get is a solution and the solution is a completely homogeneous mixture made of two or more different two or more substances so salt and water the solution would be salt water the salt would be dissolved in the water water is a great solvent it has a great ability to be able to dissolve many different things and you're going to see
why in just a minute so why is water such a great solvent it again comes down to like many other parts of water's important properties comes down to that water is polar because water is polar it has charges so if you think about salt or sodium chloride right remember that chloride is going to be negative and sodium is going to be positive so the partially positive hydrogens right so here's our partially positive hydrogens on the water is going to surround the negatively charged chlorides because opposites attract the partially negative oxygens on the water will surround
sodium so what ends up happening is water is going to form these hydration shells these shells like that they're gonna coat each of these ions and so they're gonna pull apart the salt crystal little by little by forming these hydration shells and that pulls apart that salt crystal salt when not in an aqueous solution so meaning when salt is dry it's very strong however if you take salt and you put it in water that bond is not very strong anymore the water is going to form those hydration shells and it's going to pull the salt
apart now if you've ever been like me when you get sick if you get like a sore throat and you gargle warm salt water if you've ever gone to make salt water warm salt water you've probably experienced at some point if you pour in too much salt if i pour in a whole bunch of salt is all of that salt going to dissolve in that water the answer is no think about why that is because if i pour in an excess of salt if i pour in more salt than there is water available i've reached
what's called saturation there's no longer enough water molecules to pull that salt apart and so not all of the salt is going to dissolve so now you know if you make salt water and you don't get all the salt to dissolve it means that you have more salt relative to the water and the water is not is no longer able to dissolve the salt because there's not enough water molecules present to pull that salt apart and so ionic compounds are going to break down in water and that's because water is polar so we have a
term for if water can interact with a particular substance if we call something hydrophilic hydro refers to water philic is loving so if we call something hydrophilic it's water loving this is a substance that has an affinity to water so that means that it's able to interact with water these are substances that can hydrogen bond or interact with the water and examples of things that are hydrophilic would be ionic compounds like salt for example right because salt can interact with the water or polar molecules sucrose for example which is table sugar so if you've ever
taken you know sugar and put it in your coffee the sugar is going to dissolve that's because the sugar is polar it has what we call hydroxyl groups these oh groups all over it oxygen and hydrogen different electronegativities those hydroxyl groups will make that sugar be polar and therefore that part of the water molecule that's polar can interact with the polar parts of the sugar because again you have charges and those charges can interact with one another so basically hydrophilic substances are things that have some sort of charge whether it's a full charge in an
ionic compound or a partial charge with polar molecules if there's any charge it should be able to interact with water and therefore would be hydrophilic things that are hydrophobic this is where when you start to learn the roots of the words it's going to make biology so much easier for you so we said hydro refers to water think about what phobic means if i'm arachnophobic what does that mean it means that i have a fear of spiders if i'm claustrophobic i have a fear of small spaces so hydrophobic is water fearing these are substances that
do not interact with water they're excluded they are repelled by the water and that's because these substances cannot hydrogen bond with the water what are things that are hydrophobic this would include things that are nonpolar and non-ionic again if they don't have a charge they can't interact with water so oil for example oil or fats are primarily hydrocarbons we said hydrocarbons carbon and hydrogen similar electronegativities therefore when they share they will share but they'll share equally and the molecule doesn't have a charge if that lipid doesn't have a charge it's not able to interact with
the water and therefore it's going to be repelled so again going back to cooking if you've ever taken water and you pour oil in you know that the oil doesn't dissolve the oil stays in these little pools in the water because the oil is hydrophobic it's non-polar it's not able to interact with the water and therefore it's not going to be dissolved in the water question for you you add sugar to black coffee and the sugar dissolves thus the coffee is the blank and the sugar is the blank is it red solute for the first
blank solvent for the second yellow solvent solute green polar covalent bond non-polar covalent bond blue nonpolar covalent bond polar covalent bond or purple ionic bond and hydrogen bond so again this will be a question that will be on the practice quiz so we are going to kind of finish off our chemistry lecture with acids bases and ph so a ph scale ph stands for potential of hydrogen and it's used to describe whether a solution is acidic or basic so basically a ph scale is a measurement of the concentration of hydrogen ions when we measure our
ph scale ph scale typically ranges from 0 to 14. 0 being like the most acidic 14 being the most basic or alkaline if we're looking at the concentration of hydrogen ions so here is my hydrogen ion concentration when you see brackets around hydrogens that means that's the concentration of hydrogen ions so now think of water for a minute so let's say i have my water molecule right and so water remember is polar we have oxygen and two hydrogens remember that oxygen is more electronegative than hydrogen so sometimes the electron gets stolen meaning the oxygen is
going to fully take the electron from the hydrogen so water is going to dissociate it's going to break down and this bond is going to get broken because the electron goes to the oxygen and what you end up with is a hydroxide which is an o h minus notice when we write hydroxide the minus is over here on the right but what you want to realize is that in actuality the electron the negative is actually on the oxygen it's just we write it oh minus so that's a hydroxide and that is going to separate from
the hydrogen ion because this h that's left when it gave up its electron all that's left is a proton so water can dissociate into hydroxide ions and hydrogen ions so when we talk about a neutral ph neutral ph is a ph of seven and notice that is when the concentration of hydrogen ions is equal to the concentration of hydroxide ions because pure water when it breaks apart you're going to have an equal amount of oh minus and an equal amount of 8 plus so pure water we're not talking tap water but pure water has a
ph of seven that's what we call a neutral ph the concentration of hydrogens is equal to the concentrations of hydroxides now when you go numbers less than seven that is increasing your hydrogen ion concentration and that's because the hydrogen scale the hydrogen ion concentration is a log scale it's logarithmic and so when we talk about a ph 0 the concentration of hydrogen ions is 10 to the 0 power if we talk about a ph of 1 10 to the negative 1 ph of 2 10 to the negative 2 10 to the negative 3 and so
on and so forth so notice that as my ph number goes up so we'll put a note so increase ph equals a decrease in the hydrogen ion concentration because as my ph number goes up notice so as i go from 0 to 1 to 2 to 3 my hydrogen ion concentration goes down the exponents are negative so 10 to the negative 1 is a bigger number than 10 to the negative 2. so as my ph number is going up hydrogen ion concentration is going down they are what we call inversely related as one goes up
the other goes down that is also inversely related with the hydroxide ion concentration so as hydrogen ion concentration goes down my hydroxide ion concentration up so notice that the more basic the solution the more hydroxide ions you have the more acidic the solution the higher the hydrogen ion concentration so if we look at some examples you do not need to memorize these examples of what is the ph of lemon juice i don't care that you know that okay but this is just to give you some examples of things that are acidic or basic so stomach
acid is very acidic when we get to our digestion lecture we will talk about why that's important lemon juice grapefruit juice wine tomato juice urine is slightly acidic milk is slightly acidic if we look at things that are increasing in alkaline meaning they're more basic ammonia bleach for example these would be things that are more on the alkaline side one of the things that you want to be aware of is that because the ph scale is logarithmic each change in ph unit is a 10 fold change in hydrogen ions so a ph with a solution
of 9 is 10 times as basic as a solution with a ph of 8. so now let's make sure you understand this so if i am comparing a ph 4 versus ph 6. so i'm comparing ph4 versus ph six which one is more acidic so we'll ask that first which one is more acidic answers ph4 which one has the higher hydrogen ion concentration ph4 because the lower the ph number the higher the hydrogen ions now the next question i'm going to ask is how much more hydrogens does a ph4 have versus a ph six now
oftentimes if i were to ask this question students want to say well between four and five is ten and between five and six is ten so a lot of students want to say well ph 4 has 20 times more because each change is 10 fold but what you need to realize is that you don't add those you multiply so ph 4 to 5 is 10 fold ph 5 to 6 is 10 fold so it's 10 times 10. so basically the way you can write this is 10 to the second power that's the difference between four
and six so ten to the second power is the same as a one with two zeros if i were to compare ph oops sorry ph8 versus ph2 ph8 versus ph2 which one has more hydrogen ions answer ph2 right because the lower the ph number the higher the hydrogen ions how many more hydrogen ions does the ph 2 have relative to the ph 8. so if you need a little bit of time to think about that just pause your video and then when you're ready push play but ph 2 versus ph 8 10 to the 6th
power that exponent is the difference between them so 10 to the sixth power that is a one with six zeros pen's being a little weird sorry about that so it's a one with six zeros so a million ph two has a million more hydrogen ions relative to a ph of eight think about if you were to compare ph 0 versus ph 14 10 to the 14th power that is how much more hydrogens a ph 0 has relative to a ph 14. so each change in ph is equal to a 10 fold change in hydrogen ions
so if we say that a solution is an acid an acid is a substance that releases or adds acids add h plus to solution and they increase the hydrogen ion concentration so that's because they will add hydrogens to the solution so an example of an acid would be hydrochloric acid hcl that's because if you think about hydrogen and chlorine do they have similar electronegativities no not at all chlorine is very very electronegative so if you take hydrochloric acid and you put it in water for example chlorine is more electronegative than hydrogen chlorine is going to
take the electron from hydrogen and it's going to form a chloride ion and a hydrogen ion so when you add that hcl you're going to increase your hydrogen ion concentration because you're putting in h plus into the solution as a result the ph is going to be less than 7 because remember that a ph of 7 means that the hydroxides are equal to the hydrogens if we add more hydrogens the ph is going to go less than 7. so acids are going to make the ph go down bases on the other hand are going to
do the opposite bases are substances that release hydroxides into the solutions and that will decrease the hydrogen ion concentration and this one sometimes is a little more tricky for students to comprehend about why hydroxides make hydrogens go down so i'm going to draw this out for you so if we have sodium hydroxide naoh remember that sodium has one valence electron oxygen is very electronegative so when you put sodium hydroxide into water the oxygen is going to take the electron and it's going to form hydroxides and the sodium gave up its electrons so that's why you
get sodium ions so when you add in naoh into the solution so let's say let's add a little more naoh in here and you have to remember that if we're talking about water water is going to already have some hydrogens and some hydroxides and if it was pure water the amount of hydroxides will be equal to the amount of hydrogen so notice that i drew three hydroxides one two three and i drew three hydrogens and so if i add in sodium hydroxide and i increase the ohs in the solution those ohs are going to bond
with free hydrogens in the solution so notice they're going to form a bond and when they form the bond now the hydrogen ion concentration is lower i had three free hydrogens before now i only have one so by adding in hydroxides by adding in those ohs those hydroxides are going to interact with free hydrogens they're going to form water and that is going to decrease your hydrogen ion concentration so notice that your ph is going to go up and it's going to go up to 7 above 7. so that is your base so your base
you're adding in hydroxides for example and when you add in those ohs the hydroxides are going to interact with the free hydrogens and that will decrease your hydrogen ion concentration when we talk about ionic compounds ionic compounds will dissociate in water but they don't release hydrogen ions or hydroxides and therefore they don't they don't change the ph they simply act as electrolytes they're used for salt balance but they're not going to affect your ph so again if you put sodium chloride into water it's going to break apart to sodium ions and chloride ions but that's
not going to release h plus or hydroxides and therefore it's not going to affect the ph so these ionic compounds are not going to affect the ph like an acid or a base would so just a review about which of these solutions is an acid a base or a neutral you can push pause to work through this yourself and then when you're ready turn it back on to hear your answers so the one on the left is that an acid a base or a neutral solution it's an acidic solution because it has more hydrogen ions
relative to hydroxides what about the one in the middle that is going to be a neutral solution hydrogen ions are going to equal the hydroxides so that's neutral and then the last one we have more hydroxides relative to hydrogens so that is going to be a basic solution so the last part of this is going to be talking about what are called buffers and a buffer is a solution that is able to resist change in ph so it's able to keep the ph constant and the way that it does that is that if there are
not enough hydrogens in the solution the buffer is going to donate meaning it's going to put back h plus that are no longer available or if hydrogen ions are in excess so if we now have too much hydrogens the buffer is going to accept those hydrogen ions it's going to take them away and when it does that it's going to keep the ph constant so a buffer is a solution that is going to resist a change in ph it's going to be there to help keep the ph constant and that's because the cells are very
sensitive to ph changes they can only tolerate a small window of phs so like blood for example ph of blood is 7.2 to 7.4 if you go too far outside of that it's going to start to damage cells and you're going to see when we talk about macromolecules that the reason that those macromolecules become damaged is because if the hydrogen ion concentration is affected if we get more h plus in the solution for example those hydrogens those free hydrogens that are now in that solution are going to interact with proteins for example and proteins are
held together by a variety of different bonds if you now add hydrogens to the solution it causes proteins to do something that we call denature the proteins start to unfold and if the proteins are not folded properly they're not going to function properly and the cell can't function properly so cells can be very sensitive to ph changes and the way that cells adapt and try and keep the ph constant is by having these buffers and these buffers are there to keep the ph constant if there's too many hydrogens the buffer is going to take them
away if there are not enough the buffer is gonna donate hydrogens so this is a demo that i will do in zoom talking about how buffers work it's easier to see a demonstration for this so i will do this in zoom to under to explain how buffers work and then lastly in zoom you are going to discuss this with classmates i want you to go through and fill this in this is going to help you study your terminology for the chemistry chapter so atoms have positively charged what so what part of the atom is positively
charged and then it says number present equals and they gave you the next one which is the atomic number of the element so that helps you fill in a atoms have neutral and then you're going to fill in b number may differ d would be this so when you write this out you can keep it in this format you could write it on a paper like a equals what word it is b equals this one thing i want to point out is sometimes more than one term might fit in a box and that's okay that
is completely possible that more than one term might be a possibility just put one that you came up with and i will discuss some of the other options if you didn't think about those so this is just to review your terms for chem for your chemistry and so this will conclude the chemistry lecture
Related Videos
1:52:57
Chapter 1- Chemistry Part 2 (Macromolecules)
Dr. Julie Wells
22,279 views

27:55
Chapter 1 Matter, measurement, & problem s...
Chemistry Professor
4,459 views

1:11:06
Chapter 1 - Part 1 - Introduction to Micro...
Eric Row
64,618 views

9:06
How I Passed Microbiology With An A: Pre-N...
Sukaina Attar
79,810 views

7:53
The Periodic Table: Atomic Radius, Ionizat...
Professor Dave Explains
3,819,890 views

18:49
GENERAL CHEMISTRY explained in 19 Minutes
Wacky Science
1,536,176 views

1:03:56
Chapter 2 - The Chemistry of Microbiology
Edward Kerschen
138,851 views

18:14
Basic Chemistry Concepts Part I
ThePenguinProf
1,638,465 views

52:37
Chapter 1 Introduction to Microbiology
Edward Kerschen
569,655 views

3:33
The Chemical Bond: Covalent vs. Ionic and ...
Professor Dave Explains
1,438,385 views

28:26
Brief Review of Funeral Science Part 1- Ch...
Benjamin Schmidt
45 views
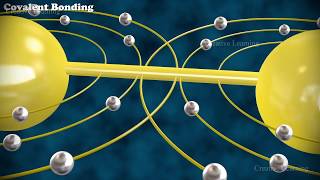
5:26
Mastering Chemical Bonding: Explained with...
Creative Learning
1,006,059 views

41:18
CHEM 101 Lecture 1.1 What is Chemistry
Lemieux Chem
6,998 views
1:07:21
Chapter 1 - Introduction: Matter and Measu...
Pablo Gonzalez
103,426 views
![The Periodic Table of the Elements in Chemistry - [1-2-12]](https://img.youtube.com/vi/CTwua04Ykso/mqdefault.jpg)
56:59
The Periodic Table of the Elements in Chem...
Math and Science
123,933 views
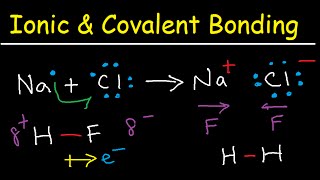
12:50
Introduction to Ionic Bonding and Covalent...
The Organic Chemistry Tutor
2,094,790 views

22:38
Lab 1-3: Aseptic technique
Dr. Julie Wells
33,223 views

19:15
Electron Configuration
Richard Louie Chemistry Lectures
574,229 views

38:15
01 - Introduction To Chemistry - Online Ch...
Math and Science
3,263,206 views