Overview of Illumina Sequencing by Synthesis Workflow
2.25M views541 WordsCopy TextShare
Illumina
Explore the Illumina next-generation sequencing workflow, including sequencing by synthesis (SBS) te...
Video Transcript:
The Illumina sequencing workflow is composed of 4 basic steps– sample prep, cluster generation, sequencing and data analysis. There are a number of different ways to prepare samples. All preparation methods add adaptors to the ends of the DNA fragments.
Through reduced cycle amplification, additional motifs are introduced, such as the sequencing binding site, indices and regions complementary to the flow cell oligos. Clustering is a process where each fragment molecule is isothermally amplified. The flow cell is a glass slide with lanes.
Each lane is a channel coated with a lawn, composed of two types of oligos. Hybridization is enabled by the first of the two types of oligos on the surface. This oligo is complimentary to the adapter region on one of the fragment strands.
A polymerase creates a complement of the hybridized fragment. The double stranded molecule is denatured and the original template is washed away. The strands are clonally amplified through bridge amplification.
In this process the strand folds over and the adapter region hybridizes to the second type of oligo on the flow cell. Polymerases generate the complimentary strand forming a double stranded bridge. This bridge is denatured, resulting in 2 single stranded copies of the molecule that are tethered to the flow cell.
The process is then repeated over and over, and occurs simultaneously for millions of clusters resulting in clonal amplification of all the fragments. After bridge amplification the reverse strands are cleaved and washed off, leaving only the forward strands. The three prime ends are blocked to prevent unwanted priming.
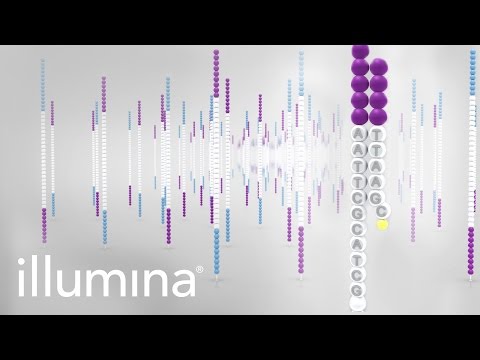
Sequencing begins with the extension of the first sequencing primer to produce the first read. With each cycle, fluorescently tagged nucleotides compete for addition to the growing chain. Only one is incorporated based on the sequence of the template.
After the addition of each nucleotide the clusters are excited by a light source and a characteristic fluorescent signal is emitted. This proprietary process is called Sequencing-by- Synthesis. The number of cycles determines the length of the read.
The emission wave length, along with the signal intensity, determines the base call. For a given cluster, all identical strands are read simultaneously. Hundreds of millions of clusters are sequenced in a massively parallel process.
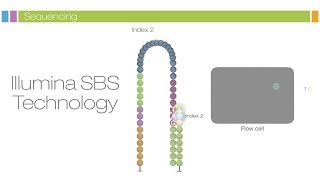
This image represents a small fraction of the flow cell. After the completion of the first read, the read product is washed away. In this step the index 1 read primer is introduced and hybridized to the template.
The read is generated, similar to the first read. After completion of the index read, the read product is washed off, and the three prime ends of the template are deprotected. The template now folds over and binds the second oligo on the flow cell.
Index 2 is read in the same manner as index 1. Polymerases extend the second flow cell oligo forming a double stranded bridge. This double stranded DNA is then linearized and the three prime ends are blocked.
The original forward strand is cleaved off and washed away leaving only the reverse strand. Read 2 begins with the introduction of the read 2 sequencing primer. As with read 1, the sequencing steps are repeated until the desired read length is achieved.
The read 2 product is then washed away.
Related Videos
4:44
Next Generation Sequencing (Illumina) - An...
Henrik's Lab
593,288 views

7:38
Next Generation Sequencing - A Step-By-Ste...
ClevaLab
320,929 views

5:04
Illumina Sequencing Technology
Illumina
871,928 views

4:09
Illumina sequencing – How it works
INTEGRA Biosciences
12,351 views
41:35
Learn about Illumina's Next-Generation Seq...
Illumina
78,079 views

25:05
Next Generation Sequencing 2: Illumina NGS...
iBiology Techniques
161,483 views

6:51
Secuenciación por Síntesis (Illumina): Con...
Brandon Ortiz Casas
94,829 views
43:32
Illumina | Introduction to Sequencing Data...
Illumina
25,646 views

51:48
Illumina Sequencing Overview: Library Prep...
Ambry Genetics
177,956 views

15:54
Método Illumina de secuenciación masiva de...
Tópicos Nucleicos
20,485 views
4:23
Intro to Sequencing by Synthesis: Industry...
Illumina
333,513 views

47:18
NextSeq 550: Run Quality and Best Practices
Illumina
5,433 views

36:09
The Beginner's Guide to RNA-Seq - #Researc...
Applied Biological Materials - abm
142,892 views

31:26
Next Generation Sequencing 1: Overview - E...
iBiology Techniques
465,100 views

33:29
NGS Data Analysis 101: RNA-Seq, WGS, and m...
Applied Biological Materials - abm
81,804 views

14:37
Sanger DNA Sequencing, From Then to Now.
ClevaLab
56,147 views

18:26
StatQuest: A gentle introduction to RNA-seq
StatQuest with Josh Starmer
497,475 views

12:04
Illumina MiSeq System: How to Start a Run
Illumina
26,245 views

1:23:46
Next-Generation Sequencing Technologies - ...
National Human Genome Research Institute
204,682 views

58:07
Real-Time PCR in Action
USDA Animal and Plant Health Inspection Service
304,303 views