Electrolysis
2.55M views5040 WordsCopy TextShare
Tyler DeWitt
Electrolysis is a process where you use electrical energy (electricity) to make a chemical reaction ...
Video Transcript:
let's talk about the chemical process of electrolysis I wanted to try to write the simplest definition that I could so here's what I came up with electrolysis is a process where electricity is used to make a chemical change happen that wouldn't happen otherwise so we have some kind of a chemical change that doesn't happen on its own but then we can use electricity to make that chemical change happen now electrolysis is often used to take a compound and break it apart into the elements that make it up we'll look at two examples of that in
this video first we'll look at sodium chloride being broken apart by electrolysis into sodium metal and chlorine gas and just FYI this is an unbalanced equation then we'll look at an example of water being broken apart using electrolysis into hydrogen gas and oxygen gas so let's get started by taking a look at this reaction so here's unbalanced equation for the electris of sodium chloride now sometimes people ask me why is a cl2 and not CL well that's because CL chlorine is one of the datomic elements these are the diatomic elements and the diatomic elements always
form groups of two they always pair up together you never find Just One of These atoms alone by itself so this would never just be CL it would be cl2 instead so the equation we've got here is an example of an oxidation reduction reaction electrons are moving between atoms in this equation I want to understand how they're moving and where they're moving and in order to do that we got to write oxidation numbers for the different elements in this equation so check it out I got some information here that will help us write oxidation number
for these elements we can do this really quick so NaCl here is an ionic compound sodium and chloride na is in group 1 a so it is always plus one okay CL chloride is one of the halogens here usually minus one positive with oxygen definitely minus one here and then on this side of the equation we have sodium and chlorine on their own they're Elements by themselves they're not bonded to any other elements so na over here is going to be zero and cl2 over here is going to be zero as well okay so those
are the oxidation numbers for these elements now to understand how the electrons move we got to take a look at the changes in these oxidation numbers we'll use this information to help us out so let's take a look at sodium here na on this side of the equation it's plus one and then over here it's zero so its oxidation number goes down so that means that it is undergoing reduction it is gaining electrons so sodium is reduced here from + one to zero and then CL goes from minus1 here to zero over here so its
oxidation number is going up it is undergoing oxidation and it is losing electrons it's being oxidized from minus1 to zero so sodium is reduced gaining electrons and chloride is oxidized losing electrons now there's something really important about this process and that's that this process doesn't happen on its own sodium chloride is also known as table salt and you could keep table salt in your kitchen for hundreds of years and it's never going to separate out into sodium and chlorine gas it's just not going to happen this process doesn't happen on its own by itself to
use chemistry terms we can say that this process is not spontaneous spontaneous process is something that happens on its own now a big part of the reason why this isn't a spontaneous reaction is because sodium and chloride over here are really happy with the number of electrons that they have sodium doesn't want to gain more electrons and chloride here doesn't want to lose electrons they're totally set right here so we have to force this process to happen and that's where the electricity part of electrolysis comes into play the electrical energy from a battery can force
this process to happen so here's our battery and a battery has these two sides the positive and negative the positive side of the battery pulls electrons in and the negative side of the battery pushes electrons out so these two things can force this process to happen so chloride doesn't want to lose its electrons but you know what the positive side of the battery just pulls electrons away from it right it's like you don't want to lose these electrons tough I'm just going to pull them away from you and the negative side of the battery that
pushes electrons out it's going to push electrons to sodium sodium doesn't want to gain electrons too bad the negative side of the battery is going to push electrons to the sodium making this process happen so now I want to show you the device that we use to do electrolysis and make this process happen and then I want to use our Atomic Vision to see the atoms and electrons and how they move during this electrolysis process so here's a picture of the device that we use to make this process happen this is called an electrolytic cell
and it's got a couple of different parts first we got this container that's full of the sodium chloride then we got a battery and the two sides of the battery are hooked up to what we call electrodes the electrodes put electrons into the sodium chloride and they pull the electrons out and then finally the sodium chloride here it's not just like the powdered table salt that you're used to from the kitchen this has got to be molten liquid melted sodium chloride sodium chloride doesn't melt until about 1500° fahr or 800° C so it's got to
be super super hot and this device has to withstand really high temperatures the sodium chloride has got to be molten for the electrolysis to happen now let's zoom in on this a little bit more if we could zoom in to the sodium chloride millions and millions of times this is what we'd see the sodium chloride is molten or liquid which means the sodium and the chloride ions have come apart from each other and they're in constant motion moving around in this container now here are the electrodes this is that one and this this is that
one these electrodes in the diagram here are way way way closer together than they should be based on this this is definitely not to scale but I wanted you to be able to see both of the electrodes now there's another important part of this electrolytic cell and that is the battery the diagram that I put here shows the direction that electrons move electrons move from here into the battery and then out of the battery into this electrode so we can give names to these two electrodes based on how the electrons are moving okay the electrode
over here we call the anode the anode is the site of oxidation oxidation is happening in here because the anode is pulling in electrons because this is the direction the electrons move now over here we have the cathode reduction happens to the cathode the cathode pushes out electrons because this is the direction that electrons are moving from the battery so we got the anode and the cathode we got the battery the electrodes and the sodium chloride we're ready to do some electrolysis I'm going to get rid of a few of these ions to make the
process a little bit clearer okay that's a little bit clearer so now we hook the battery up to the electrodes and this is what happens so this cathode here is connected to the negative side of the battery so that means that it's going to have a negative charge these positively charged sodium atoms are going to be attracted to the negatively charged cathode because opposite charges attract so we got this na+ moving over to the negatively charged cathode and electrons as we said are being pushed out of the battery into the cathode and this electron is
going to be given to the na+ ion and gaining that electron is going to get rid of the charge on that na so it's going to turn this into an neutral sodium atom the same thing is going to happen to this one over here it's got a positive charge it's attracted to the negative cathode moves over here an electron gets pushed from the battery it goes into the sodium ion and gaining that electron causes the sodium to lose it charge so now we have two neutrally charged sodium atoms now over here at the anode the
opposite thing is going to happen electrons are going to be pulled out of these ions so we got this chloride over here it's attracted to the anode because the anode is hooked up to the positive side of the battery and this is negatively charged so it's going to be pulled over here and the anode is pulling in electrons so one of the electrons from the CL minus is going to be pulled up towards the battery and that is going to cause the negative charge to go away because it's lost one of its electrons and then
over here this CL minus is also going to get attracted to the anode it's going to lose one of its electrons it's going to get pulled up here towards the battery and that is going to cause this to lose its negative charge now we said that chlorine here is one of the datomic elements so that means that we're never just going to find one of these atoms on their own as soon as they don't have a charge anymore they're going to pair up to form a cl2 molecule this is gas and it's actually going to
float away so that's how the electrons move in this electrolytic cell here's a diagram of the process that I just showed you I want to use this to write half reactions for what's going on at the anode and the cathode so oxidation happens at the anode and reduction happens at the cathode let's write these half reactions so over here at the cathode we start out with na 1+ that gains an electron plus e minus the symbol for electron and that gives us neutral sodium na without a charge that's how we know it's neutral now over
here at the oxidation half reaction I'm going to write this a little bit incorrectly first and then I'm going to fix it okay so here we start out with two cl1 minus okay two CL minus and then what happens is these lose two electrons one two get pulled up to the battery so I'm going to write minus 2 e minus that's incorrect but I'll fix it in a minute and that gives us a molecule of cl2 to gas now what's wrong about this well you can't subtract things in a chemical equation even though it makes
a lot of sense so to make this correct I have to put these two electrons on the other side of the chemical equation so here is the correct version where I've just moved the electrons over here and this is the correct way to write the oxidation half reaction now that I've written the oxidation and reduction half reactions I want to show you how we can put them together to get a reaction for this whole process now let's combine these half reactions that we just wrote In order to add or combine these half reactions together they
have to have the same number of electrons but they don't right now there's one electron here and two electrons here so to fix that I'm going to take this reduction half reaction and I'm just going to multiply it by two just like it's a math equation and that is going to give me this new reaction action I've just put a two in front of everything Distributing it across the reaction okay so there's my new reduction half reaction and then here's my oxidation half reaction now that they have the same number of electrons I can add
them together and this is what I get I take everything on this side of the arrow and put it on one side I have my na my two electrons and my 2 CL on this side and then on this side I have my na my cl2 and my 2 e minus now there's still a little bit more that I have to do before this equation is totally finished if there's anything on both sides of the arrow we cancel it out so there two electrons here and two electrons here I'll cancel those out and then I
have 2 na+ here and I have two CL minus here I can combine those together to make NA so my final equation is going to look like this 2 NAC and then on this side 2 na plus cl2 and you'll notice that I've also put in these parentheses to show the physical state of each of these particular compounds this is liquid because it's so hot it's molten the na is also liquid because it's so hot and the cl2 is gas and just floats away so let's just pull everything together that we talked about here is
our final equation for the the electrolysis of sodium chloride this happens because sodium gains electrons and is reduced this happens at the cathode in the electrolytic cell which pushes out electrons it's where reduction takes place and it's where sodium gains these electrons here is the half reaction for the reduction of sodium then chloride loses electrons it undergoes oxidation and this happens at the anode in the electrolytic cell the anode pulls in electrons oxidation happens there and here is the half reaction for chloride losing electrons and getting oxidized so that's how sodium chloride turns into sodium
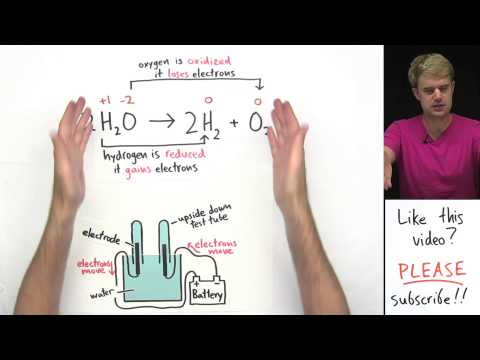
and chlorine gas during electrolysis here's another Super common example the electrolysis of water we take water H2O add some electrical energy and we get high hen and oxygen gas you might be able to tell this is a balanced equation because I think you guys can handle it now what's with H2 and O2 here well hydrogen and oxygen just like chlorine which we saw earlier are examples of the datomic elements you'll never find just an H or an O alone by itself these elements always pair up and form twos so that's why we got H2 and
O2 here now this equation represents an oxidation reduction process so electrons are moving they're being transferred let's figure out how they're being transferred by looking at the oxidation numbers I've got some rules for writing oxidation numbers here these are just a few of them these are just the ones that are relevant to the equation that I'm talking about here okay so I've got hydrogen hydrogen is + one when it's with non-metals like oxygen oxygen is usually -2 and then over here we have hydrogen and oxygen these are elements by themselves they're not combined with any
other elements so H2 is going to be zero and O2 is going to be zero okay so those are the oxidation numbers and now to see how the electrons are being transferred let's look at the change in oxidation number okay starting here with hydrogen hydrogen is + one and then over on this side of the equation it is z Z so its oxidation number is going down which means that it is undergoing reduction it is gaining electrons Ox uh oxygen over here is min-2 and then on the right side of the equation it is zero
so Oxygen's oxidation number is going up from min-2 to 0 oxidation number going up that means it is undergoing oxidation which is loss of el electrons so oxygen is being oxidized it is losing electrons here this process is important because just like we saw in the previous example with sodium chloride this process doesn't happen on its own it's not spontaneous you can have water in a glass for hundreds of years if it doesn't evaporate and it's not going to turn into hydrogen gas and oxygen gas it's just not going to do that on its zone
so just like the previous example we're going to have to use the electrical energy from a battery to force this process to happen so now let's take a look at the device that we'd use to do electrolysis of water it looks a little bit different than the device we talked about earlier because often when we do electrolysis of water we want to be able to keep or hold on to the oxygen gas and the hydrogen gas that get produced so this is the electrolytic cell that we'd use to do the electrolysis of water here's what's
going on we have a container that has water in it and then we got these weird looking things here what are these well these are actually test tubes that are filled with water and are put upside down in the top of this container so it's like you'd fill them with water and then you'd very carefully turn them upside down so they're still filled with water and they're in the container then just like in the previous example we've got these electrodes here the electrodes are in the test tubes you can see that they're like sticking in
the test tubes and the electrodes are hooked up to the battery it's the electrodes that are putting electrons into the water or pulling electrons out of the water okay and then finally of course we have the water now I should say this electrolysis process doesn't just happen in distilled pure water we got to add a little something like like like sulfuric acid here to allow electricity to flow through the water so you can't just do this in pure water got to add what we call an electrolyte like h2so4 sulfuric acid okay now let's look at
how these electrodes here are connected to the battery okay this electrode here is hooked up to the negative side of the battery so electrons as you can see here are moving out of the negative side and they're being pushed into this electrode so that means that this is the cathode it's the site of reduction over here this electode is hooked up to the positive side of the battery so electrons are moving out of it that means that oxidation is going to be taking place it's going to be pulling electrons from things and this is what
we call the anode so we've got hydrogen gas and oxygen gas what gets made where well well hydrogen here in water gets reduced to form hydrogen gas so the cathode the site of reduction is going to be where hydrogen gas gets produced H2 produced here at the cathode and then oxygen in water gets oxidized to form oxygen gas so over here at the anode the site of oxidation oxygen is going to be produced so we hook everything together we add little sulfuric acid to the water connect the electrodes to the battery and we'll start seeing
bubbles that's the gas being formed on the electrodes and those bubbles are going to start moving up to the top of the test tubes and if you look carefully you see a really cool thing happen the level of the water starts to go down because gas is flowing up it's floating up and it's collecting at the top of the test tubes and it's forcing the water down so you see the water level slowly move down as the gas collects at the top of the test tubes it's really cool and you can watch this you can
see this happen in the lab now something else really interesting happens you'll see what I did here and I I'm not just being careless on the side that's producing hydrogen you get twice as much gas as the side that's producing oxygen there's twice as much hydrogen gas as oxygen gas why is that well take a look at this balanced equation here okay we have 2 H2 and just 1 O2 so there is a 2: one ratio of hydrogen gas to oxygen gas so that means that when we do this electrolysis we are physically going to
get twice as much much hydrogen gas in this test tube as we get oxygen gas over here okay so that's the big picture of how we do electrolysis in the lab and what's going on here in the electrolytic cell now let's take a look at the half reactions for the reduction of hydrogen and the oxidation of oxygen let's break this process down into the two half reactions that make it up now let me just tell you when it comes to writing half reactions for the electrolysis of water there are so many different ways to do
it and it seems like every textbook or every teacher has their own way of writing these half reactions in this video I'm using the half reactions that I think make most sense but if you're teacher or textbook has a way they really want you to do it instead just learn their method every way of writing these is essentially equivalent they're essentially the same they just look a little bit different so again I'm using the reactions that I think make most sense for this video let's start with a reduction of hydrogen I really want to show
you what's going on with the atoms and electrons in these half reactions okay so we start with two molecules of water H2O and you'll see that I've written oxidation numbers above each of the atoms so in the reduction of hydrogen essentially what happens is it's like we take one hydrogen from each of these water molecules and pull them off and then we combine them to make a molecule of H2 hydrogen gas now what has to happen with the electrons for this to take place well each of the hydrogens over here has an oxidation number of
plus one but then they have an oxidation number of zero over here so that means that each of these hydrogens has to gain one electron so that its oxidation number can be bumped up by one okay here's here's a diagram sort of showing what happening here okay this hydrogen was plus one and then it gains an electron and becomes zero this hydrogen was plus one and it gained an electron and became zero where do these electrons come from in the electrolytic cell well they came from the cathode because the cathode is what pushes out electrons
the cathode is where reduction happens okay so we get one molecule of H2 but then we have these parts of the water molecule which you're just left behind so we get an O and an h and these two things together have a negative charge of one minus these are polyatomic ions are called a hydroxide ion and the reason why they have a charge here is because notice that their oxidation numbers haven't changed because we're not adding or removing electrons from the rest of the water molecule so these numbers are just staying the same as they
were over here you add these oxidation numbers up and you getus oneus one so that's the charge of this ion okay so this is how the reduction happens with pictures let's look at how we can actually write an equation actual half reaction for what's going on here we start with two molecules of H2O then we gain two electrons so plus 2 e minus and that gives us one molecule of H2 and then get two o minus so that's the half reaction here that's a half reaction for the reduction of hydrogen now let's take a look
at the half reaction for the oxidation of oxygen okay just like up here we start with two molecules of H2O and for the oxidation of oxygen it's going to be like we pull off these two oxygen atoms to make a molecule of o2 oxygen gas now how about the electrons in this case well both of these oxygen were minus two for their oxidation number over here and then they became zero over here so each one of them has to lose two electrons to become zero this one was minus two then it loses two electrons become
zero this one was minus two it lost two electrons to bump its oxidation number up it's losing electrons to zero so that's what's going on here now where are these electrons going well the electrons are going to the anode because it's the anode and oxidation reduction reactions that is pulling in electrons okay so this is what's happening with the oxygen what about the rest of this well we have four hydrogens left over after we pull off those oxygens and their oxidation numbers are not changing because we're not adding or removing electrons so we're just going
to get four H+ over here left over that have an oxidation number of plus one because that's not changing let's do the same thing that we did up here let's write a half reaction for the process this is a little bit more challenging as before I'm going to write it incorrectly first and then we're going to fix it okay so we start with 2 H2O and then we lose four electrons -2 -2 so -4 electrons and that gives us O2 + 4 h plus these up here now as you probably know we can't use the
subtraction sign in a chemical equation so we have to make this four electrons and put it over here so this is what we're going to yet instead we just move this four electrons over here 4 e minus there we go okay there we go now we can see it and these are the half reactions for the reduction of hydrogen and the oxidation of oxygen now let's combine them put them together okay now so for combining these half reactions we want them to have the same number of electrons the reduction half reaction that only has two
electrons we have four here so the way we're going to solve this is we're going to take this whole equation and we're going to multiply it by two and distributing this two across the equation we are going to end up with this 4 H2O four electrons 2 H2 and 4 oh minus so that is our new reduction half reaction now we can take these two half reactions and add them together we're going to get this we take everything from this side of the equation and put it here 4 H2O 4 4 e minus 2 H2O
then we have our arrow and then we have 2 H2 4 oh minus O2 + 4 h++ 4 e minus all right now there are a few things that we can do here if something appears on both sides of the uh of the arrow here we can cancel it out okay so we'll get rid of this one and we'll get rid of this one but that's not all we can do look at this we have four o minus here and we have 4 H+ oh minus and H+ combine to make water now also look at
this we have four H2O here and 2 H2O here so we can combine these to get six H2O all right so here's how we can rewrite it we pull these together and get 6 H2O we get rid of the electrons there and then we have 2 H2 we combine 4 oh minus and 4 H+ to get 4 H2O and then we have our oxygen but you know what there's actually one more thing we can do because look we have H2O on both sides of the equation so I can subtract it so we can get rid
of it from one side I'm going to do Min -4 H2O here I'm going to do minus 4 H2O here that's completely going to get rid of the H2O on this side and it's going to leave me with 6 - 4 2 H2O on this side here is the same balanced equation that we used when we started you put the half reactions together and this is what you get so that is the electrolysis of water hydrogen is reduced it gains electrons this happens at the cathode the sight of reduction and H2 is produced there then
oxygen is oxidized it loses electrons this happens at the anode the site of oxidation where the oxygen is produced we got these two electrodes in these upside down test tubes and the gas the hydrogen and oxygen collects at the top and we've seen that we get twice as much hydrogen gas as oxygen and that's just because in our balanced chemical equation there's a ratio of two hydrogens to one oxygen so that is why we get twice as much hydrogen here as oxygen here so that's how electrolysis works that's how you put electrical energy into a
chemical reaction to make something happen that wouldn't happen otherwise something like taking sodium chloride and breaking it apart into sodium metal and chlorine gas or something like taking water and splitting it apart into hydrogen gas and oxygen gas
Related Videos
15:17
Electroplating
Tyler DeWitt
510,746 views
23:35
Galvanic Cells (Voltaic Cells)
Tyler DeWitt
1,849,311 views

16:37
Introduction to Electrochemistry
Tyler DeWitt
1,852,999 views

13:05
Introduction to Oxidation Reduction (Redox...
Tyler DeWitt
5,238,288 views

30:03
DIY Hydrogen/Oxygen Generators From Grocer...
NightHawkInLight
4,326,106 views
16:00
Half Reaction Method, Balancing Redox Reac...
The Organic Chemistry Tutor
1,376,382 views

14:56
Balancing Chemical Equations Practice Prob...
Tyler DeWitt
7,162,626 views
27:42
Introduction to Galvanic Cells & Voltaic C...
The Organic Chemistry Tutor
719,507 views
9:04
Electrochemistry: Crash Course Chemistry #36
CrashCourse
2,236,364 views

13:12
Electrolysis of Water - Electrochemistry
The Organic Chemistry Tutor
481,053 views
16:05
Oxidation and Reduction Reactions - Basic ...
The Organic Chemistry Tutor
2,388,451 views
18:00
How to Balance Redox Equations in Basic So...
Tyler DeWitt
2,183,129 views

5:11
What Is Electrolysis | Reactions | Chemist...
FuseSchool - Global Education
2,450,373 views

43:36
Making the stinkiest chemical known to man
NileRed
14,369,025 views

6:21
Electrochemistry
Professor Dave Explains
317,384 views

16:58
Introduction to Limiting Reactant and Exce...
Tyler DeWitt
2,906,477 views

15:00
How to Balance Redox Equations in Acidic S...
Tyler DeWitt
1,680,676 views

37:51
Making metal crystals from Pepto-Bismol
NileRed
15,537,556 views

17:15
Testing INSANE chemistry recipes from a 19...
styropyro
3,959,948 views

4:10
Voltaic cell | How does it work?
Sabins
299,521 views