How Small Is An Atom? Spoiler: Very Small.
9.39M views741 WordsCopy TextShare
Kurzgesagt – In a Nutshell
Atoms are very weird. Wrapping your head around exactly how weird, is close to impossible – how can ...
Video Transcript:
Atoms are ridiculous and unbelievably small. A single human hair is about as thick as 500,000 carbon atoms stacked over each other. Look at your fist, it contains trillions and trillions of atoms.
If one atom in it were about as big as a marble, how big would your fist be? Well… about the size of Earth. Hm… still hard to imagine?
Let’s try something different Look at your little finger. Imagine that its tip is as big as the room you’re sitting in right now. Now fill the room with grains of rice.
One rice corn represents one cell of your fingertip. Now let’s zoom in on the rice corn. And now, one cell is as big as the room you’re in right now.
Let’s fill it with rice again. This is about the size of a protein. And now, let us fill all the empty spaces between the rice corns with fine grains of sand.
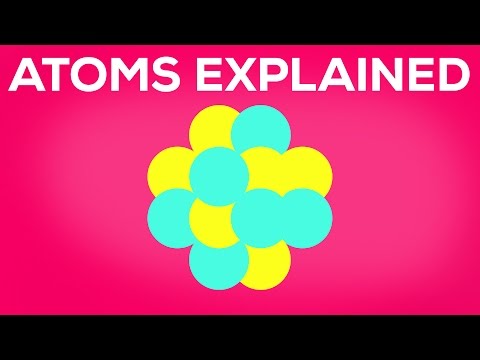
This is roughly how small atoms are. What is an atom made of? Let us just pretend that atoms look like this for a minute to make it easier to understand.
An atom consists of three elementary particles: neutrons, protons and electrons. Protons and neutrons bind together and form the atom core, held together by the strong interaction, one of the four fundamental forces in the universe. They are made from quarks and held together by gluons.
Nobody knows exactly how small quarks are. We think they might literally be points, like in geometry. Try to imagine them as being zero-dimensional.
We suspect that quarks and electrons are the most fundamental components of matter in the universe. Electrons orbit the atom core. They travel at a speed of about 2,200 km/s, fast enough to get around the Earth in just over 18 seconds.
Like quarks, we think electrons are fundamental particles. 99. 999999999999% of an atom’s volume is just empty space… Except that it isn’t.
What we perceive as emptiness is actually a space filled by quantum fluctuations, fields that have potential energy and build and dissolve spontaneously. These fluctuations have a fundamental impact on how charged particles interact. But that’s a topic for another video.
How much space do the core and electrons actually fill? If you were to subtract all the spaces between the atom cores from the Empire State Building, it would be about as big as a rice corn. All the atoms of humanity would fit in a teaspoon.
There are extreme objects where states like this actually exist. In a neutron star, atom cores are compacted so densely that the mass of three Suns fits into an object only a few kilometers wide. By the way, what do atoms look like?
Well, kind of like this. Electrons are like a wave function and a particle at the same time. We can calculate where an electron might be at any given moment in time.
These clouds of probability, called orbitals, are where electrons might be with a certainty of 95%. The probability of finding an electron approaches 0 the further we get away from the atom core, but it actually never is zero, which means that, in theory, the electron of an atom could be on the other side of the universe. Okay, wait a second.
These strange thingies make up all the matter in the universe. For many dozens of known elements, you don’t need many dozens of elementary particles, just three. Take one proton and one electron, and you have hydrogen.
Add a proton and a neutron, you have helium. Add a few more, you get carbon, a few more, fluorine, even more, gold, and so on. And every atom of an element is the same: all hydrogen atoms in the universe, for example, are the same; the hydrogen in your body is exactly the same as the hydrogen in the Sun.
Do you feel confused right now? We certainly do! Nothing on this scale of the universe makes any sense in our world, and we’ve not even begun talking about quantum mechanics or the particle zoo, which are even stranger!
Our model of atoms has changed a number of times since we first conceived it, and the current one will certainly not be the last. So let us support scientists and research and wait for the next wave of mindboggling new information about this strange world that is the basis for our existence. Subtitles by the Amara.
Related Videos
12:15
What Is An Atom And How Do We Know?
Stated Clearly
3,221,835 views

5:34
What Is Something?
Kurzgesagt – In a Nutshell
7,809,434 views

6:16
Fusion Power Explained – Future or Failure
Kurzgesagt – In a Nutshell
14,423,324 views
5:11
Atoms As Big As Mountains — Neutron Stars ...
Kurzgesagt – In a Nutshell
8,244,836 views

32:44
The Simple Math Problem That Revolutionize...
Veritasium
7,757,007 views

8:38
VFX Artist Reveals the True Scale of Atoms
Corridor Crew
9,719,499 views

16:43
How Are Quasiparticles Different From Part...
PBS Space Time
544,990 views

7:31
Emergence – How Stupid Things Become Smart...
Kurzgesagt – In a Nutshell
8,936,866 views

5:28
Just How Small is an Atom?
TED-Ed
7,615,057 views

6:21
What is Dark Matter and Dark Energy?
Kurzgesagt – In a Nutshell
11,952,479 views

32:46
Electrolysis
Tyler DeWitt
2,581,087 views

33:07
A Brief History Of Atom | Democritus to Qu...
Klonusk
258,879 views

4:52
How Small is an Atom?
Jared Owen
1,914,121 views

19:55
The Surprising Map of Plants
Domain of Science
1,271,809 views

13:33
Black Hole's Evil Twin - Gravastars Explained
Kurzgesagt – In a Nutshell
3,733,564 views

21:37
What Makes The Strong Force Strong?
PBS Space Time
1,205,622 views

30:31
The Basic Structure of the Atom | Chemistr...
The Great Courses
689,598 views

11:08
Fever Feels Horrible, but is Actually Awes...
Kurzgesagt – In a Nutshell
6,984,899 views

18:07
Can We Create New Elements Beyond the Peri...
PBS Space Time
794,383 views

31:48
The Map of Particle Physics | The Standard...
Domain of Science
1,584,538 views