The Origin of the Elements
2.71M views10554 WordsCopy TextShare
Jefferson Lab
The world around us is made of atoms. Did you ever wonder where these atoms came from? How was the g...
Video Transcript:
Tonight we have with us Ed Murphy from UVA. He's an astronomy professor out there. He's also in charge of the night events at the McCormick Observatory and, if you go to their website, you will notice that two nights of the month they do have public nights at the McCormick Observatory out there at UVA and it is a nice opportunity. So, please join me in welcoming Ed Murphy. [applause] Thank you very much. And I'd really like to thank JLab for having me out here tonight. It's a great thing that the Hampton area has such a fantastic
science education and outreach group that you have here at JLab because they just do wonderful stuff. I was here this past summer working with them on a workshop for teachers, so lots of things go on here that are just really fantastic. But, I thought what we would talk about tonight is the origin of the elements and the fact that everything around us is made of atoms. We all learn in elementary school that the atom is sort of the basic building block of matter. You're made of atoms. I'm made of atoms. This table up here is
made of atoms. And the question is "Where did these atoms come from?" And, in particular, there's a couple atoms that I think are especially important to talk about. And, one of those is gold. We all carry a little piece of gold with us wherever we go. Or, I should say, the vast majority of us. So, for many of us, it's our rings that we wear. If you're wearing a pair of glasses that have gold rims, there's probably a little bit of gold plating on those. You might be wearing an earring that has some gold in
it. If you're not convinced that you have any gold jewelry on, then you might be carrying gold if you're carrying a cell phone because gold is an excellent conductor of electricity and it's contained in almost all modern electronics. And, so, you're carrying a little bit of gold in there. If you don't have any jewelry and a cell phone with you, you might not be carrying any gold, but they you're likely carrying some mercury with you. If you've breathed air in the last day, and I see everybody here has, then you've got some mercury inside of
you. It comes from, in many cases, coal fired power plants that are further west from us. And, as we burn that coal, it releases mercury into the air, which ends up landing on our food and our water. So, where did these heavy elements come from on the periodic table? So, what we're going to talk about tonight is the origin of gold, element number 79 right there. Right next to it is element number 80, mercury, on the periodic table. But I also want to talk about some elements that are a little more personally interesting to us.
And those are the elements that you are made of. And the first question I have for the audience tonight is "What are you mostly made of?" Water. Most people are made of mostly water. The vast majority of the mass in our body is water. What element is water mostly made of? You think hydrogen. So, you'll remember the chemical formula for water is H2O. That's two hydrogen atoms and an oxygen atom. So, for most people, would say that you're made mostly of hydrogen, because, if you're mostly water, and there's two hydrogen atoms and one oxygen atom
in water, you'd say you're mostly made of hydrogen. But, the thing is, astronomers, and, in fact, most scientists, think a little bit differently because we think about the amount of mass that goes into making an element and not the number of atoms of that element. So, if you look at those periodic tables that you could have picked up on the way coming in, you'll notice that hydrogen has an atomic mass of one. So, those two hydrogen atoms in water have a total atomic mass of two. Oxygen, on the other hand, has an atomic mass of
sixteen. And, so, by mass, you are mostly made of oxygen. What about this room? What is this room mostly made of? What's the most common element in this room, do you think? It's a little bit tougher one. But, if you think about the walls for a second, because those are probably some of the most massive things in here. I'm going to guess the walls are mostly made of concrete. Concrete is, in large part, made of sand. Sand is silicon dioxide. And silicon dioxide is a silicon atom and two oxygen atoms. The silicon atom has a
mass of 28. The two oxygen atoms have a mass of 32. So it turns out the walls, as well, are mostly made of oxygen. It turns out that oxygen is probably the most common element in this room by mass. It's certainly the most common element in you. And many people find that surprising. We often think, when we study astronomy, that oxygen is one of the rarer things that's out there. But, in fact, oxygen makes up about 65% of your body. After oxygen, carbon is the next largest thing. And then hydrogen, a little less than 10%.
Nitrogen, about 3%. And then all those other things make up just a couple percent down here. Calcium, phosphorus, potassium. And they make up just a few percent of a human body. So, you're mostly made of oxygen. Something that we often think of as being rare, but, in fact, as we will find out, is quite common in the universe. Astronomers not only know what you are made of, but we can tell what things in space are made of. Even things that we have never visited and things that we are not likely to ever visit. Take this
star cluster for example. This is a newborn cluster of stars. Young, hot, blue stars, right here, that have just been born. This is the cloud of dust and gas from which they were born. Astronomers can analyze the light from these stars, and the gas around them, and we can tell what these stars are made of. Even though, with our current technology, it would takes us millions of years to get to these stars. And the thing is, even if we do, even if our children develop spacecraft that can go ten times faster than our current spacecraft,
it will still take them millions of years to get to this cluster of stars. If their grandchildren do a factor of 100 better than that, it will still take hundreds of thousands of years to get out to this cluster of stars. We are never, not in the next thousand years or longer, going to sample these stars. Never the less, astronomers can tell you exactly what these stars are made of. And, in fact, they're 74% hydrogen, 25% helium and everything else is 1%. So, all the oxygen, the nitrogen, the carbon, the iron, the nickel, the gold.
Everything. All those elements are 1% and the stars are 74% hydrogen, 25% helium. Turns out, that's the same composition as our sun. 74% hydrogen, 25% helium and 1% everything else. And astronomers determine what distant things are made of by breaking the light of those objects down into its component colors. We know that if you take white light and pass white light through a prism you get a whole spectrum of colors. What scientists discovered back in the 1800s is if you take special elements, particular elements, and you excite them to glow, either by heating the up
or running electricity through them, when they glow they give off only particular colors of light. So, if you make hydrogen glow, for example, hydrogen gives off a particular color of red light, a particular color of blue-green light, and two blue lines. Helium. If you excite helium to glow, gives off particular colors of light. And, you'll notice the colors that helium gives off are different than the colors that hydrogen gives off. You can think of them as a fingerprint or a bar code. Each element in the universe glows with its own particular set of colors. And
all hydrogen atoms, regardless if they are on earth or in deep space, glow with these colors. But, only hydrogen glows with those particular colors. So, when we're faced with a distant object and we want to know what it's made of, we can take the light from that object, break it into its component colors, and analyze the specific colors that we see, and use those to determine what the object is made of. We can to the same thing for our sun, for example. This is a spectrum of our sun. And, when we break our sun's light
down, we get the full rainbow here. But, now we see particular colors are missing in the spectrum. And those particular colors that are missing happen to correspond to hy- In this case, this red line right here, happens to correspond to hydrogen atoms in the atmosphere of our sun that are absorbing that particular color of red light. That red color right there that's being absorbed by the atmosphere of our sun is the same as that red color right there. This blue-green color right here is the same as that one right there. So these lines happen to
come from hydrogen atoms in our sun. And astronomers can determine the composition of objects using a variety of different telescopes. Telescopes like the Large Binocular Telescope which is located on Mount Graham, a couple hours northeast of Tuscon, Arizona. I bring this picture up just to mention to people that the University of Virginia is a partner in this telescope. It's the largest telescope in the world on a single mount. And the people of Virginia own a share of this telescope. And the University of Virginia, in particular, has built a spectrograph, one of these devices for breaking
light into its component colors, that's used on that telescope. But we can also use other telescopes like the Hubble Space Telescope. And, in particular, one that I've worked on, is the Far Ultraviolet Spectroscopic Explorer. The name tells you everything you need to know. It looks at ultraviolet light, not visible light that we can see with our eyes. Spectroscopic means that it breaks the light into its component colors. And Explorer means that it was a small satellite mission, not one of the big flagship missions like Hubble, but a smaller telescope mission. We cannot only use visible
light and infrared light and ultraviolet light, we can even use radio waves to determine what things are made of. Just a few hours west of Charlottesville, so probably about 5 hours west of us here, in Green Bank, West Virginia, is the world's largest fully steerable radio telescope. And this radio telescope picks up radio waves from space and different molecules in space emit different radio waves, including water, for that matter. And we can use radio telescopes like this to trace the distribution of the different atoms through our galaxy and through the universe. So, let's go back
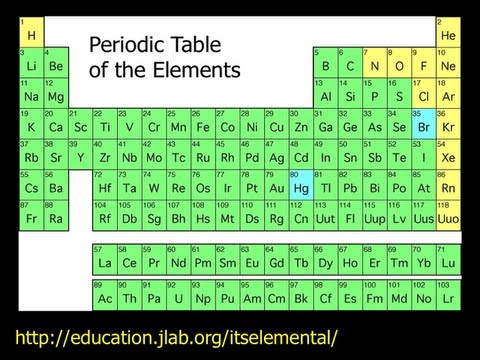
to the periodic table of elements for a few minutes. I should mention that I took this periodic table of elements from the JLab webpage. There's a really nice It's Elemental page where you can click on each of the elements and get information about each of the elements And so, um, you should visit that web page after the talk tonight. But, there's a couple things I'd like to tell you about the periodic table of elements and... Maybe the most amazing thing I can tell you about the periodic table of elements, and it's really quite a remarkable
thing to be able to say. The periodic table of elements is not just the elements that we know of. It is all the elements that things can be made of in the universe. There is nothing that stuff can be made of that is not on our periodic table of elements. And that's, it's an amazing thing to be able to say, that if there's something in the universe and it's made of matter, it is on our periodic table of elements. And, so, it's worth explaining why that is for a few seconds, to try and justify that
statement. It often comes up, for example, when people talk about UFOs. They say if a UFO landed, we would know it wasn't from this world because it would be made of stuff that we don't have on our periodic table of elements. And the answer is, if it's made of matter, it'll be made of stuff that's on our periodic table. It has to. Because, it turns out, there's nothing missing off our periodic table. The first thing to know about the periodic table is each element has a number up there. So, hydrogen is element number one. Helium
is two. Lithium is three. Beryllium, four. Boron, five. On and on up the periodic table of elements. That number tells us the number of protons inside the nucleus. There are three basic building blocks of matter. There are protons, with a positive charge. Neutrons, with no change. And they're contained in the nucleus of the atom. And then there are the negatively charged electrons that orbit around the atom. And they orbit at quite a distance away from the nucleus. The nucleus is remarkably small. The best way to think about the nucleus is it's about the size of
a pea in the middle of a football field. So, if you imagine the size of a football field with the electrons out here representing the edge of the football field, then the nucleus is about the size of a pea down there at the center of the football field. So the nucleus is very small compared to the distribution of atoms. The different elements on the periodic table have different numbers of protons. So, we mentioned before that hydrogen was element one and it's element one because, down in its nucleus, deep down at the center, it has one
positively charged proton. And, which means it also has one negatively charged electron orbiting around it. Helium is element number two on the periodic table. Its atomic number is two because it has two positively charged protons. Regular helium also has two neutrons. And, so, it has an atomic mass of four, but its atomic number is two. Carbon has atomic number six because there are six positively charged protons down there. So, if we look back at the periodic table of elements, the element number tells us the number of protons. So, we have one proton. Two protons. Three
protons. Four. Five, six, seven, eight. As you go down the periodic table of elements, you'll see that there are no numbers missing from the periodic table. So, for example, let me pick this. Forty-one, forty-two, forty-three, forty-four, forty-five. Each number is represented on the periodic table. And, the thing about protons are, is they don't come in halves. There's no such thing as forty-five and a half protons. You either have forty-four protons or forty-five protons. You don't have something in between. And, if you look carefully at the periodic table, you will see that there are no numbers
missing on the table. We have discovered every element there is on the periodic table. I'll take about these big ones in a second. Now, it's important to say that that has not always been true. When the periodic table was first invented, there were holes in the periodic table. This is the father of our modern periodic table, Mendeleev. And Mendeleev, when he put together the first version of the periodic table, he organized it in rows instead of columns. But, the important thing is, you'll notice, that there were some question marks here. He understood that the periodic
table was telling us something about the way matter is put together. And it wasn't just a convenient way for humans to organise all the different elements. It really was telling us something about the structure of the atom. And, what he discovered was holes in the periodic table. And these holes corresponded to elements that hadn't yet been discovered. And, when I told you about elements forty-two, forty-three, forty-four here on the periodic table, I didn't pick those by random. Element number forty-three is a beautiful example. It's called technetium. There used to be, until the 1920s, a hole
in our periodic table. There was no technetium on the periodic table. And that's because there's no stable version of technetium. Every version of technetium is radioactive and it decays into something else. If you build, and we can do it today. We can make technetium today. If you make it today... The most common version disappears within a few days. It's highly radioactive. And it disappears within just a few days. If you look in the rocks on the surface of the earth, which are hundreds of millions or billions of years old, if there was any technetium in
there, it long ago decayed and disappeared. So there was a hole in the periodic table. But today, we can take element forty-two, molybdenum, and bombard it with neutrons, and we can make element forty-three, technetium. So much so that some of you may have ingested, or had injected into you, technetium. It's commonly used in medical imaging today. If you've ever had a radioactive tracer test, about 80% of all radioactive tracer tests today are done with technetium. So, we use it in medical testing today. But, for a long time, there was a hole here on the periodic
table. So, there are no holes left today. Except, you might argue, well what about these big elements that scientists always seem to be coming up with new elements, all the way up to ununoctium up here on the periodic table. The thing about these major, these giant elements down here, is that we make them by smashing together smaller elements. And, when we smash them together, for a tiny fraction of a second, they come together and they form one of these larger, more massive elements. But all of these elements are so unstable that the vast majority of
them decay is less than a second. So, even if you make one of these atoms in one of these atom smashers, it's gone in less than a second. It disappears because it's so unstable it decays into smaller atoms again. Even the most stable of these elements are not stable for much more than a second in time. So you can't really build a spaceship out of ununoctium or any of these heavy elements because they decay within a second. And so, I would argue that you can't make anything in the universe out of any of this. So,
remarkably, our periodic table has on it everything that there is in the universe. If it's made of protons, neutrons and electrons it's on our periodic table. And, if a UFO should ever land and explain all those things in astronomy I'd love to know about that we haven't figured out yet, I can guarantee, that if it's made out of protons, neutrons and electrons, it's on our periodic table. So that raises the question, where did those protons, neutrons and electrons come from in the first place? The stuff the atoms that we're made of are made of those
fundamental building blocks, where did they arise? And the answer is, they came directly from the Big Bang. The next time you're getting up in the morning. The young students won't understand this, but those of us that are my age will understand this. When you swing out of bed and you're sitting there and you're feeling kind of old and creaky that day, regardless of how old you are, remember that the protons, the neutrons and the electrons that make up your atoms are a lot older than you are. In fact, they date all the way back to
the Big Bang. And they are, in fact 13.7 billion years old. That number, the age of the universe, was determined about 10 years ago by a NASA satellite called the Wilkinson Microwave Anisotropy Probe. We'll see a picture from it here in a minute. And it's one of the hardest fought numbers in astronomy. We fought for 100 years to figure out the accurate age of the universe and now we know it. It's 13.7 billion years old. And the protons, the neutrons and the electrons in your body were made in the Big Bang. Now, the Big Bang
isn't just a hypothetical idea that astronomers have come up with. The Big Bang can be directly tested in laboratories today. In fact... This is a simulation of a collision at the giant particle accelerator in CERN, in Switzerland, on the border of France and Switzerland. They take protons and they smash protons together at high speed because when those two protons meet head-on, the pressures and the temperatures and the densities in that collision are the same as they were a tiny fraction of a second after the Big Bang. Here on earth we can recreate the conditions of
the Big Bang when we collide atoms like this in our atom smashers. In fact, that's a large reason why we do it, is to make these kinds of conditions that we don't normally experience around us. So, we understand the Big Bang fairly well. We know that the Big Bang, during the Big Bang, the universe was really hot. And it was so hot that the universe was filled with light and that light consisted of high energy photons, a kind of light called gamma rays. And, every now and then, these two gamma rays would collide with one
another. And, when they collided with one another, they would make a piece of matter and a piece of anti-matter. And, the only difference between matter and anti-matter is the charge. So, this, for example, is a proton with a positive charge and this is an anti-proton with a negative charge. And, so, when these photons would collide, they would make two pieces of matter. And, the only thing that was required for these two photons to make that matter was that they have enough energy. Einstein's famous equation, E=mc^2, tells us how much energy is takes to make a
certain amount of matter. If you know the mass of the proton and the mass of the anti-proton you can figure out how much energy it took to make them. And, it turns out, the only form of light that has enough energy to make these things are highly energetic gamma rays. Now, fortunately for you and me, gamma rays are pretty rare these days, unless you work in one of the nuclear industries or work here at JLab, you probably don't encounter gamma rays very often. But in the early universe was filled with gamma rays, this was going
on all the time. In the first few seconds of the universe, matter and anti-matter were popping into existence as these photons would collide. The problem is, is matter and anti-matter have opposite charges. Well, remember that opposite charges attract one another. The positive proton and the anti-proton are attracted and they annihilate in a burst of energy, just like in Star Trek. When matter and anti-matter come together, they annihilate and you get back those two photons of light. If this was the only way to make matter in the universe, then every time we made a piece of
matter, we'd make a piece of corresponding anti-matter. But, for reasons physicists don't understand right now, every now and then in the early universe, the universe made a piece of matter, but it did not make the corresponding piece of anti matter. Or, it made the anti-matter, and the anti-matter was so unstable that it decayed away and all we're left with is the matter. But this is the matter, the protons that you and I are made of. They were made by light in the early universe and those protons are the ones that remain because their partners either
weren't created or decayed away long ago. The physicists, or the scientists, who answers the question about why the anti-matter wasn't made sometimes or decayed away, without a doubt, will win the Nobel Prize in physics. So. So, my generation has been working really hard on figuring out this problem. We haven't gotten it yet, so maybe one of the students in here will finally be the person who figures out why it is that that anti-matter wasn't made and only matter was left over. Because, today, in our universe, there's essentially no anti-matter. Hardly any, in the universe as
a whole. Well, for the first... few minutes in the big bang, the first three minutes, it was so hot that all the protons and the neutrons that were created start colliding with one another and they start undergoing nuclear reactions. And neutrons and protons can collide to make a heavy form of hydrogen. And that heavy form of hydrogen can collide with neutrons to make an even heavier form of hydrogen. Or, that can collide with a proton to make a form of helium. This hydrogen and that proton can combine to make another form of helium. In the
first three minutes of the Big Bang the universe was hot enough that these protons and neutrons could collide with one another and start building up the elements on the periodic table of elements. And we get the formation of hydrogen atoms, which are easy, because hydrogen atoms are just a single proton. But we get the formation of helium and lithium on the periodic table. But, there's a problem in the first three minutes. The universe is rapidly expanding. It's expanding so fast and it cools off so quickly that just three minutes after the Big Bang the nuclear
reactions shut down. It gets too cold for nuclear reactions. So, the universe does not have a lot of time to make elements because its only got three minutes of nuclear reactions to do that. On top of that... it turns out that just after lithium on the periodic table of elements there's a bottleneck. The next element on up from lithium, the form of beryllium that would be made, is unstable. So unstable that the universe can't make it. This is what the periodic table of elements looks like today. This is what the periodic table of elements looked
like three minutes after the Big Bang. There were only three elements in the whole universe. Hydrogen, helium and lithium. And I only include lithium here, there was a tiny, tiny amount of lithium in the early universe, so I include it here because there was some lithium made. But, three minutes after the Big Bang, the universe was 75% hydrogen and 25% helium. There was no gold. There was no carbon, no nitrogen, no oxygen. Nothing. I like to point out to students that chemistry class was a lot easier back then because there was just hydrogen and helium
and helium is a noble gas, so it doesn't form molecules. The problem was there could be no chemistry class because there's no carbon and oxygen to make the chemist. Steve's web page that has the, uh, has the periodic table of elements was a lot easier to build back then. But, there could be no Steve to build it because there was no carbon or nitrogen or oxygen to make Steve. So that was the Big Bang in the first three minutes. So, where did the gold come from? Because the gold didn't come from the Big Bang. The
carbon and the oxygen in your body did not come from the Big Bang. So, where did they come from? Well, we have to trace this hydrogen and helium gas over time and see what happens to it. So the next step after the Big Bang... is that the universe continues to expand. And, at first, this gas is very evenly distributed, very smoothly distributed throughout the universe. But gravity starts clumping it together. And as gravity starts clumping this gas together we can see clumps forming in the early universe. This is a picture of the glow left over
from the Big Bang. This is a whole sky image. It's like one of those odd earth maps where they and show you the whole earth map so they disassemble the earth and they spread the whole thing out. So this is a map that shows you the whole globe of the Big Bang over the whole sky. The light that you're seeing - it was taken by the Wilkinson Microwave Anisotropy Probe, that NASA satellite I mentioned a minute ago, and this is the glow that was released 380,000 years after the Big Bang. But you'll notice that just
380,000 years after the Big Bang, matter is already clumping together. Gravity is already starting to pull it. And we can run simulations starting with a very smooth universe and just let gravity run in our simulations, and we can see how the universe starts gathering into clumps. And we go from the very smooth universe that we had early on, to the very clumpy universe we have today. And any deep, deep image of the universe, like the extreme deep field that was recently released by the Hubble Space Telescope, the deepest image that humanity has ever taken of
the universe, shows us that the universe today is very, very clumpy. The gas isn't evenly distributed. It's clumped together. And the basic building block of the universe is the galaxy. This is a picture of what our Milky Way galaxy would look like if we could get outside the galaxy. But, as I said before, we've never been outside our galaxy. Our children will not get outside the galaxy and their great-great-grandchildren will not get outside the galaxy. Because it would take hundreds of thousands of years to travel out to this distance to look back, even travelling at
the speed of light. And we're a long way from doing that right now. So, this is another galaxy, NGC 4414, that we think looks like our Milky Way galaxy. If we could get outside the Milky Way and look back on it, this is what our galaxy would look like. Our galaxy consists of a few hundred billion stars and giant clouds of gas and dust. And those giant clouds of gas and dust are where new stars are being born. Now, we don't see the Milky Way like this, we see the Milky Way like this because we
live inside of it. It's like a giant pizza with a grapefruit at the middle. The grapefruit is this big bulge down here, the pizza is the disk of material. But, because we live inside the pizza, in the middle of the pizza, about halfway out from the center, when we look over towards the constellation of Sagittarius, we see the grapefruit down at the center and then here is the rest of the pizza around us. Everything you can see in the night sky, with one exception, is part of our Milky Way galaxy. These stars are in the
Milky Way. They just happen to be the stars that are above us. These stars are in the Milky Way. Those are the stars that are below us. These are the stars that are in the Milky Way that happen to be all around us. And, of course, if you think about a thin pizza, if you live in the middle of a pizza, there's not much pizza above you, not much pizza below you, but lots of pizza around you, which is why our galaxy looks like a line cutting through the sky. In fact, the only thing that
you can see with the naked eye that is not part of our galaxy is a faint, fuzzy object in the constellation of Andromeda, right here. And that faint, fuzzy object is another galaxy called the Andromeda Galaxy, about 2.4 million lightyears away from us. And, I'm going to guess that here in the Hampton area you're not going to see that because of all the light pollution, that you'll need to get out under dark, country skies in order to see the Andromeda Galaxy. Inside of galaxies, the basic building block of the universe are stars. And stars are
the next part of our story as what happens to the elements. Stars are giant balls of hydrogen and helium gas. Our sun is a giant ball of hydrogen and helium gas. It's about 300,000 times the mass of the earth. It's 109 times the diameter of the earth, so the earth would be a little, tiny spot up here compared to the size of the sun. But the sun has no solid surface. It is a gas all the way to the center. And it is, as I said earlier, 74% hydrogen, 25% helium and 1% everything else. Down
at the center of the sun, because of the weight of all these layers of gas pushing down on the center, as they push down on the center, they compress that gas. That gas is really hot down there. Its got a temperature of about 15 million degrees. And there are nuclear reactions going on down there and that is what powers our sun. And they're two basic nuclear reactions in the universe. There are fission reactions, where you take big elements, like uranium, and you break them into krypton and barium and release a bunch of neutrons. Those are
fission reactions. And there are fusion reactions, where you take light elements, like hydrogen, and collide them together to fuse, or build, bigger elements. Now, this one's important to us. Fission is important because this is how a lot of our electricity is generated. A fair bit of the electricity that's running the lights in this room and this projector are coming from nuclear power plants in Virginia. In central Virginia, we have the Lake Anna nuclear power station and, at Lake Anna, they're taking uranium atoms, they're splitting them apart, and, in the process, it's releasing energy, and that
energy's getting turned into electricity. We also make nuclear weapons out of this. Uranium bombs and plutonium bombs are fission weapons. Fusion, on the other hand, where you take the light elements and combine them to build bigger elements, is something that we have not yet mastered, at least for generating electricity. We have, however, mastered this, if you can call it mastering it, in nuclear weapons. Hydrogen bombs. We can do this reaction in an uncontrolled way, but we haven't yet figured out how to control it and harness it and turn it into electricity. But we understand fission
and fusion nuclear reactions quite well. And you can guess which one powers the sun because I've already said that the sun is 74% hydrogen. And, so, the sun is made mostly of hydrogen and it's fusion reactions that power the sun. Deep down, inside the sun, in the core of the sun, but only in the core of the sun, not in the outer parts of the sun, just deep down in the core, it's hot enough, about 15 million degrees, where you can collide protons together and build up heavier elements. This is the set of nuclear reactions,
the chain of reactions, that goes on deep down inside the center of the sun. What happens in the sun is a hydrogen atom, which is a single proton, which has a positive charge, and another hydrogen atom, a single proton with positive charge, collide with one another. Now, they don't want to come together because, remember, like charges repel one another, so a positively charged proton and a positively charged proton - Normally, when you try to drive them together, they're positive - both positive charges will repel one another and they'll pull apart. But, if you this under
high enough temperatures, like the 15 million degrees that we have down at the center of the sun, you can drive them together so forcefully that they get so close together that the two of them can react and form something heavier. A form of heavy hydrogen. And that heavy hydrogen can combine with another proton to make a form of helium. Two of those combine to make another form of helium. The ultimate result in our sun is that four hydrogen atoms go in and one helium atom comes out. So hydrogen is converted to helium in our sun.
Each and every second inside of our sun 600 million tons of hydrogen are being converted to helium in our sun, every second. Now, you'll remember from chemistry class in school that, in chemical reactions, mass is conserved. You start with a certain number - amount of mass to begin with, that mass has to be conserved. That's no longer true with nuclear reactions. In the case of nuclear reactions, it's the combination of mass and energy that are conserved. This helium atom that comes out has less mass than the 4 hydrogen atoms that went in. It's a tiny
fraction. It's 0.7% less mass than the 4 hydrogen atoms that went in, but that 0.7% of matter was turned into energy. And, according to Einstein's E = mc^2, that matter that went missing comes out in the form of energy, and that is what powers the sun. So, our sun is converting hydrogen to helium. This is a problem for our sun. Or, at least the long term future for our sun. Because our sun is like your automobile. It's running on fuel and, someday, our sun will run out of fuel. This shows what our sun looked like
when it was born. This is the distance from the center of the sun on out to the surface of the sun. And this is the composition of the sun. So, this is the core, this is the surface. You can see that throughout the sun, when it was born - at birth, the sun was mostly hydrogen and a little bit of helium. Today, the sun is four and a half billion years old. So, it's about half-way through its life. The sun has used about half of the hydrogen in its core and converted that to helium. Our
sun is about half-way through its life. It is a middle aged star. When the sun is about 10 billion years old it will have converted almost all of its hydrogen into helium. And, at this point, our sun will start to die, because there will be no longer any nuclear reactions going on down at the center, and our sun needs those nuclear reactions because gravity is trying to pull all that gas together. And the nuclear reactions are providing the force that balances against the force of gravity. Without those nuclear reactions, gravity will win and the center
of our sun will start to contract. And, as the center of our sun contracts, it releases so much energy that it puffs up the outer layers of the sun into a red giant star. So, the ultimate fate of our sun is to become a red giant star. This is the size of our sun today. This is the size of our sun when it's a red giant star. It will get enormous compared to its size today. But, what's contradictory about it is the center of our sun is actually shrinking. The center is shrinking and getting smaller
and the outer layers are getting puffed out. And, in fact, that center will eventually become unstable. And, when it becomes unstable, it will blow off those outer layers. In its lifetime, our sun is converting hydrogen into helium. When it's a red giant star, at the end of its life, that core will get hot enough that it can actually convert helium atoms into carbon and some carbon into oxygen on the periodic table. It can only do that later on because, right now, it's not hot enough at the center of the sun to convert helium into carbon.
The problem with helium is it has two protons. Another helium atom has two protons. That's plus two and plus two. It's a lot harder to drive those together. Where as it takes a temperature of about 10 million degrees to fuse hydrogen into helium, the temperature has to be over 100 million degrees to fuse helium into carbon, and our sun's not hot enough yet. But it will be in the red giant phase. And it will fuse helium into carbon and a little bit of carbon into oxygen. But our sun isn't big enough and it won't do
any more than that. And that's as far on the periodic table as our elements will go. Now, keep in mind that the carbon and the nitrogen and the oxygen in your body did not come from our sun because our sun won't do this until it's dying. So, it's not as if it did this long ago. This is what the sun's going to do at the end of its life. And this is what's going to end up - this is the ultimate fate of our sun. This little white dot at the middle is a white dwarf
star. It's the burned out core of a star like our sun. And, this beautiful planetary nebula that you see around it, this glowing nebula, is the outer atmosphere of the star. The dead core becomes unstable and it puffs off those outer layers and creates this beautiful planetary nebula right there. The other problem with stars like our sun is all the carbon and the nitrogen and the oxygen, it stays locked up in that white dwarf star. It doesn't return those elements back out into space to be used by future generations of stars. So, the carbon, the
nitrogen and the oxygen in your body did not come from a star like our sun. The carbon, the nitrogen and the oxygen in your body actually came from a much bigger star. Giant stars undergo different nuclear reactions. And, so, let's talk for a minute about the nuclear reactions that happen in big, giant, massive stars. And, this is a really cool plot that shows you how much there is of each element in the universe. This tells you the amount of the element and this is each of the elements listed here. And, the thing to know about
this table is this is a logarithmic table, which means each one of these tick marks is a factor of ten. So, just looking at this, it looks like there's almost as much nitrogen as oxygen in the universe. But, in fact, they're separated by about one tick mark, which means there's ten times as much oxygen as there is nitrogen in the universe. So, these are the abundances of the elements. And, we've already said that hydrogen and helium are the most abundant elements, by a lot, in the universe. And then there's hardly any lithium, beryllium and boron,
but here are the other elements. And, if you look at that periodic table of elements, you'll notice something very interesting about the abundant elements. So, can someone tell me what atomic number carbon is? Six. So, what number is oxygen? Eight. What's neon? Ten. Magnesium? Twelve. Silicon? Fourteen. Do you see a pattern developing here? It's the even numbered elements. Six. Eight. Ten. Twelve. Fourteen. Sixteen. Eighteen. Twenty. Twenty-two. Twenty-four. Twenty-six. The even numbered elements in the universe are ten times more abundant than the odd numbered elements. Why is that? Why does the universe prefer the even numbered
elements over the odd numbered elements? By the way, another thing before we go on, to point out about this table is I mentioned that hydrogen and helium are the most abundant, look at what the third most abundant element in the universe is. It's oxygen. You're made of oxygen. You are made of the third most abundant element in the universe. After oxygen, you're made mostly of carbon. Carbon is the fourth most abundant element in the universe. You are made of the common stuff of the universe. Carbon and oxygen are not rare. After hydrogen and helium, they're
the two most abundant things in the whole universe. So, let's go back to this question of the even numbered elements. What is it about the even numbered elements? It has to do with the way massive stars live out their lives. Massive stars can fuse hydrogen to helium. But, when they're done fusing hydrogen to helium, they can fuse helium to carbon. And then, they can fuse - undergo other nuclear reactions where helium and carbon can make oxygen. Helium and oxygen can make neon. Carbon and carbon can make magnesium. Oxygen and oxygen can make silicon. And, if
you look at these reactions, you'll notice that the basic building block of all of them is the helium atom. Three helium atoms made carbon. Helium and carbon make oxygen. Since the basic building block of all the elements is the helium atom with number two. If you start with a building block that's two, and you put two of them together, you get four. Six. Eight. Ten. Twelve. Fourteen. Sixteen. The way massive stars live out their lives is they fuse ever heavier elements, and they're always using helium. So, silicon and helium make sulfur. Plus helium makes argon.
Plus helium makes calcium. And on, on up the periodic table until we reach iron on the periodic table. Massive stars, in their lives, will fuse hydrogen all the way up to iron on the periodic table. So, this is the periodic table today. This is what massive stars can do. During their lives, they'll fuse hydrogen into helium. And helium into carbon. Carbon to oxygen to neon to magnesium to silicon, sulfur, argon. All the way up to iron. But, iron's really special. Turns out iron is the most stable element in the universe. There's no element that's more
stable than iron. That is, iron is so tightly held together that, if you fuse these atoms to make iron, it releases energy. If you fission these atoms, break them apart to get down to iron, you release energy. But when you get to iron, there's no place else to go. If you wanted to take iron and build up heavier elements, you would have to add energy. And stars don't want to do that. Stars are using these nuclear reactions to generate energy. Having to do nuclear reactions where you have to put energy in will just suck energy
out of the center of the star. So, stars end up here at iron. And, in fact, a massive star, a really big star. Say, a star that's about twenty-five times the mass of our sun. It will fuse hydrogen to helium for about 7 million years. Our sun will do that for 10 billion years. Really massive stars, even though they have a lot more fuel available to them, they don't live very long lives because they burn that fuel so fast. It'll burn helium to carbon for 500,000 years. Carbon to neon for 600 years. Neon to oxygen
for about a year. Oxygen to silicon for about half of a year - 6 months. And then silicon to iron in one day. And the very last day of the life of this star, it starts fusing silicon into iron. And, in the last few minutes of that star's life, the inside of the star looks like a giant onion. There's a big ball of iron sitting down here at the middle. And, surrounding that, is a shell of silicon that's fusing to iron. And around that is a shell of oxygen fusing to silicon and on, on out.
As I said, this iron is so stable it can't undergo any nuclear reaction. So, without nuclear reactions, gravity is pulling that ball of iron together. Gravity's trying to contract it. The iron atoms are pushing back against the force of gravity. And, in particular, it's the electrons that are in there that are pushing back. But gravity squeezes it tighter and tighter and they're pushing back further and further. Eventually, though, this silicon shell keeps dumping more and more and more iron on that core. And that core gets more and more massive. And more and more massive. And,
eventually, it gets so massive, and the gravity is so strong, that the atoms can't push back against the force of gravity anymore and they give up. And the core collapses. And, in about two seconds, the core of this star, which is about the same size as our planet, but weighs probably two or three times the mass of our sun - So, it's many, many times the mass of the earth, but about the size of the earth, that core collapses. And, when it collapses, it releases so much energy that the star blows itself apart in a
titanic explosion called a supernova. Only the most massive stars go supernova. If you look in here, this is in a nearby galaxy called the Large Magellanic Cloud. This is a giant star forming region right here. There is a star in this picture that is about to go supernova. It went supernova in 1987. This was a picture taken a couple days before the star blew itself up. In looking at that picture, can you find the star that's ready to blow itself up? If you could, you're doing a lot better than astronomers did because we had no
idea that it was that star right there that was ready to blow itself up. So, if I go back, you can see the star. See, the thing is, with astronomy, we can see the surface of a star, but we can't see what's going on deep down inside the stars, so we had no idea that that star was ready to blow itself up. But, for a few days, that star outshone our whole Milky Way galaxy of hundreds of billions of stars. That's how much energy they give off. And, when these stars explode, the majority of the
mass of the star is blown out into space. And, all the elements that were made during the life of that star are spread back out into space as well. So, we had said that this is periodic table of elements. This is what it looked like three minutes after the Big Bang. This is what it looks like because of stars like the sun. Massive stars can build all the way up to iron. And, because they blow themselves up at the end of their lives, they return all these elements back out into space. But, remember that core
collapse, where that big ball of iron collapsed on itself in two seconds? When that big ball of iron collapses, the densities and the pressures and temperatures are so high that, in about two seconds, all the other elements on the periodic table are made. The reason that gold is precious to us is because it's rare. And the reason that gold is rare is that the universe had about two seconds to make it when the core of that star collapsed. Because, it's the only time that the pressures, the temperatures, the densities are high enough to make some
of these heavy elements like gold and mercury and silver. They're made when these massive stars implode at the end of their lives. So, let's trace the history of your atoms, and in particular, your gold atoms, from the very beginning of the universe until the present day. The atoms in your gold jewelry started out as hydrogen and helium atoms in one of these giant star forming regions, like this one, this giant cluster of stars. The first time your atoms found themselves inside of a star, they weren't deep down in the core of the star. They were
likely in the outer parts of the star. And, remember, there are no nuclear reactions in the outer parts of a star. The nuclear reactions are only in the core of the star. So the first time your atoms found themselves inside of a star, that star exploded in a titanic explosion and it blew those atoms back out into space, but they were still hydrogen and helium atoms. The second time your atoms found themselves in one of these giant star forming regions, like the Orion Nubula. Here's the belt of Orion and his sword. And that's the Orion
Nebula, a giant star forming region. The second time they found themselves in a star, they likely found themselves not deep down in the core of the star, but in the outer layers of the star. And, when that star blew up, it returned all those elements back out into space. The third time your atoms found themselves in one of these giant star forming regions it's likely that the found themselves deep down inside the core of the star. And this time, the hydrogen atoms were converted to helium. The helium to carbon. The carbon to oxygen. All the
way up to silicon. Up to iron. And then, when it exploded, it made the gold that's in your jewelry. And that gold was spread back out into space in that titanic explosion at the end of the life of the star. After that gas spread out into space, your atoms found themselves in one final star forming region, like the Eagle Nebula down in Sagittarius. The famous one with these beautiful pillars of dust sticking up. And, this is really neat to astronomers because we actually see little clumps of gas up here. And those little clumps of gas
are forming stars. And, when we look at those forming stars, this particular group of stars comes out of the Orion Nebula, we see the stars and we see, surrounding them, these black disks of dust. Those are planets in the process of forming. The last time you were in one of these star forming regions, your atoms were not in the star. They were in that disk of dust surrounding the star. And as gravity clumped those pieces of dust together, they made little rocks. Those rocks clumped together to make asteroids. Those asteroids clumped together to make planets.
And the fourth time that your atoms were in a star forming region they ended up, not in the star, but in the third planet from the sun. And, they weren't deep down inside the earth. They were actually up close to the surface. Your atoms have spent most of their time up close to the surface of the earth here. And this is where your atoms have come from. You are, as Carl Sagan said, literally made of star-stuff. The atoms in your body were forged in the furnaces of stars billions of years ago. But, your atoms haven't
been in just one star. You've probably been in at least two or three different stars in the history of the universe. And, as I said, this time, your atoms ended up, not in the star, but in the planet. The last thought I want to leave you with is "What is the ultimate fate for your atoms?" So, you're made mostly of oxygen. What's going to happen to your atoms in the future because, I hate to tell you, you don't own your atoms, you're just borrowing them for the time you're here on earth. Well, when we all
pass away, whether we're cremated or buried, our atoms become part of the surface of the earth. And, over millions of years, those atoms will be recycled through plants, through animals, through other living things on the surface. Eventually, though, our sun will die. When our sun dies, it will become a red giant. And our star will get so big - the diameter here is two astronomical units, which means the radius is one astronomical unit. An astronomical unit is the distance from the earth to the sun. Our sun will get so big that the outer layers of
the sun will come all the way out to the earth's orbit around the sun. When it does that, it may not be enough to completely vaporise the earth, but it will vaporise the outer layers of the earth. So your atoms, which are part of the outer layers of the earth, will be vaporized off the surface. They will become part of the outer layers of this red giant star. And, when it dies, our sun, you'll remember, the core shrinks down to make a white dwarf. The outer layers are blown into a beautiful planetary nubula. That is
the fate of your atoms. You will someday be a planetary nebula. And, I didn't mention it before, but I'll mention it now. See that green glow that's coming from the center of the nebula? Those are oxygen atoms. So, those are the oxygen atoms that you are made of and that's where you're headed someday. Because you'll be back out in space. And then, after billions of years into the future, these atoms will be in the next generation of stars. So, the Milky Way is like a giant recycler. Your atoms have been in stars, and they will
be in future stars again. But you have them just for this short period of time. So, thank you very much. I'm happy to take questions for a few minutes. I think we have about five minutes for questions. So, thank you. [applause] Yes. Does the city of Charlottesville have a black sky at this point in time [inaudible]? No, Charlottesville does not have a dark sky ordinance. Not a very good dark sky ordinance, anyhow. I don't know that there's too much hope for getting it in Virginia. I think really we're just going to have to work in
rural areas and try very hard to preserve the dark skies that we have in the rural areas today. But I'm not holding out much hope that there's any hope for taking cities like Charlottesville or Hampton and trying to reverse what we've already done. And we've worked, actually - I will say we've worked very hard in Charlottesville to try and get a dark sky ordinance, but there's too much push back from others. Yes. What is that grapefruit in the middle of the Milky Way? Yes, so the middle of the Milky Way, the grapefruit at the middle,
it's a big ball of stars called the galactic bulge. And those were some of the first stars to form when our galaxy was forming. And, so, if you look at it in that picture, it's just a big ball of stars sitting down there at the center of the galaxy. Really old stars. Yes. [Mostly inaudible, but something about black holes and if data are skewed if one is in the line of sight of an observation.] Okay, so the question is "How do we know, when we look at things that are far away, like millions of lightyears
away, how do we know there's not a black hole between us?" Or other objects. Well, the answer is, in many cases, there are other objects between us and we see those. So, let me go back to these. So, when we look at star forming regions like this, most of the stars that you can see here are actually foreground stars that are in the foreground. The thing to keep in mind about black holes is they are incredibly small. If we take our sun and shrink it down until it's a black hole, its radius is just a
couple kilometers, which is a couple miles across. So, that is so infinitesimally small when we talk about distances that are this big that it has no effect at all. Well, see, that's the thing. Black holes, they have this really bad reputation of sucking everything into them. And, it turns out, it's only if you get right up close to them that they suck everything in. But, an interesting thing to think about our sun is - the earth is held in orbit by the mass of our sun. If we took our sun and shrunk it so small
that it became a black hole, it turns out we haven't changes the mass of the sun, and we haven't changed the distance from the earth to the sun. So, even if our sun were to turn into a black hole, the earth would continue to orbit the sun, just like it does today. So, it doesn't suck things in. The only way that black holes suck things in is if you get really, really close to them. Close to what's called their event horizon, where gravity's very strong. But that event horizon is the thing that's just a couple
miles across. So, you have to get really close to black holes. Yes. [inaudible, but I think I heard 'black hole' in there] So, they're two things there. One is, do black holes strip matter off stars. Yes. Stars are often born in binary systems. Two stars orbiting around one another. And, in those binary systems, if the more massive one dies and becomes a black hole, when the less massive one dies and becomes a red giant, that matter can get sucked into the black hole. There's a beautiful example of this in the constellation of Cygnus. It's the
best candidate we have for a black hole. But isolated stars like our sun, which are not in binary systems - Black holes are not at all common in the galaxy. It is not common - I would say, in fact, it's remarkably unusual for an isolated star, like our sun, to have an interaction with a black hole. And the only really massive black hole in our galaxy is deep down at the center of the galaxy. And down at the center of our Milky Way galaxy there's a black hole 4 million times the mass of our sun.
But, that's 26,000 lightyears away from us and we orbit around the galaxy. We never find ourselves down there in the center. The only stars that have to worry about that black hole are the ones that are close to it. So. Can we thank Dr. Murphy again, please, for his talk tonight. [applause]
Related Videos

15:24
The Surprising Origin of All the Elements ...
Arvin Ash
312,470 views

1:01:17
Solving the secrets of gravity - with Clau...
The Royal Institution
395,560 views

1:00:11
What is the Periodic Table? How are Eleme...
Math and Science
332,966 views

31:48
The Map of Particle Physics | The Standard...
Domain of Science
1,546,918 views

15:54
The weird ways the elements got their names
RobWords
554,739 views

1:19:27
The man who tried to fake an element
BobbyBroccoli
12,369,158 views

1:11:54
How the Universe Made the Elements in the ...
Georgia Tech Physics
241,597 views

1:02:35
The Paradoxes of Time Travel
Linda Hall Library
415,447 views

51:29
The 9 Experiments That Will Change Your Vi...
Astrum
3,222,514 views

12:01
What the Maker of Ozempic Doesn't Want You...
More Perfect Union
1,740,665 views

34:16
Dark Matters: Have We Really Failed To Ide...
World Science Festival
160,427 views

26:23
The Dark Energy Delusion | Claudia de Rham...
Perimeter Institute for Theoretical Physics
354,689 views

56:11
A Brief History of Quantum Mechanics - wit...
The Royal Institution
4,214,134 views
49:06
Why is the Universe accelerating? Nobel La...
ANU TV
2,265,675 views

47:45
Cosmic Chemistry with Kate the Chemist & N...
StarTalk
351,821 views

28:28
Russell's Paradox - a simple explanation o...
Jeffrey Kaplan
7,474,041 views

49:34
Investigating the World's Elements | BBC E...
BBC Earth Science
124,271 views

1:25:34
Investigating the Periodic Table with Expe...
The Royal Institution
1,172,104 views

53:19
Einstein's Quantum Riddle | Full Documenta...
NOVA PBS Official
3,398,768 views

42:59
What Really Is Everything?
History of the Universe
3,659,906 views