Spin Spin Splitting - N 1 Rule - Multiplicity - Proton NMR Spectroscopy
594.28k views3049 WordsCopy TextShare
The Organic Chemistry Tutor
This organic chemistry video tutorial provides a basic introduction into spin spin splitting / coupl...
Video Transcript:
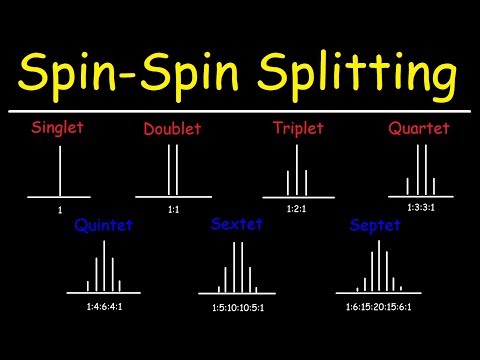
in this video we're going to go over the multiplicity of a proton nmr signal and the multiplicity is basically the number of peaks that we see in the signal so on the left here we have just one signal and there's no splitting pattern so this is just a singlet here it splits into two parts this is a doublet and below it you can see the intensity ratio of those two peaks it's a one-to-one ratio next we have a triplet which appears as three signals it's really one signal but split into three and the intensity ratio
for that is one two one if it splits into four parts you have a quartet and four five is a quintet six is a sextet and seven is a septet sometimes if you have more than four like if you have five or more you could just call it a multiplet sometimes which might be easier uh to do it that way now let's focus on the intensity ratios that we see for like a quartet or a quintet you don't have to memorize these numbers because there's a very simple way in which you can find these numbers
for the different types of signals that we can have now there's something called pascal's triangle which i'm sure you heard some point in either in high school or college when you took algebra or a pre-calc the first number is a one and then the next number the next row will have two ones so this is just the intensity ratio for singlet and this is the intensity ratio for doubling next add the two numbers one plus one is two now you're always going to have a one on the left side and on the right side so
this is the intensity ratio for a triplet now if we add one plus two that gives us three and if we add two plus one it also gives us three and put a one at the the edges of the triangle so now we have the intensity ratio for a quartet three plus three is six one plus three is four three plus one is four and now we have the intensity ratio for a quintet now four plus six is ten six plus four is ten one plus four is five so this gives us the intensity ratio
for a sex ten ten plus ten is twenty ten plus five is fifteen one plus five is six and so now we have the intensity ratio for a septa it's amazing how some of the things that you've learned in math now comes into play with uh things like this like nmr who would have known that pascal's triangle would be related to it so that's a quick and simple way in which you could find the intensity ratios for the split in patterns that you'll see in an nmr spectrum now let's work on an example problem let's
use ethyl bromide as an example and what we're going to do here is we're going to draw a rough sketch of the proton nmr spectrum for this molecule so right now we need to determine how many signals we would get in this molecule notice that there's two different types of protons the three protons in the methyl group are equivalent to each other so they will show up as one signal which we can call signal a and the two protons in the ch2 group will show up as one signal now what is the split in pattern
for the ch3 protons or protons a what is the the splitting pattern for those protons what would you say would you say it's a singlet doublet triplet quartet now there's something called the n plus one rule the m plus one rule allows us to determine what type of splitting pattern we're going to get so if we're analyzing proton a you need to look at the adjacent protons in this case proton b so n is the number of protons on the adjacent carbon so we have two protons plus one that's going to be three and three
corresponds to the signal of a tripling now let's analyze proton b so looking at the adjacent carbon there are three protons on it so n is 3 using the m plus 1 rule we have 3 plus 4 i mean 3 plus 1 which is 4. and so we're going to get a quartet with the intensity ratio one three three one so that's how you could determine what type of splitting pattern you have using the n plus one rule now in order to draw the graph we need to determine the location of these signals a ch2
group attached to a br that's going to have a chemical shift somewhere between three to four and a methyl group is usually around one ish but because of the presence of this electron withdrawn group it turns out that an ethyl bromide it's going to be shifted up field or not up field but shifted downfield to about 1.6 so let's draw the signal for the methyl group so around 1.6 we're going to have a triplet and so we're going to get something that looks like that let me use a different color my drawn is not perfect
but you get the point now for the ch2 group those protons will show a signal let's say about 3.5 and so we could show the quartet like that and so that's how you could use the n plus one rule to either draw an nmr spectrum or even identify the signals on a graph to determine which molecule corresponds to it now the only other thing we need to mention is the integration notice that we have three protons for signal a and this is for the triplet this is for the quartet to indicate the integration we could
say that the triplet corresponds to 3h and the quartet signal corresponds to 2h now what you need to understand with regard to the integration is that the number of protons the ratio between the number of protons of the different signals correspond to the ratio of the area under the curve in those signals so just keep that in mind typically the signal will with the higher number of protons usually corresponds to a signal with a greater height so to speak because the greater the area under the curve is the greater the height of the signal so
we expect that this signal will be taller than this one but technically the ratio of the protons corresponds to the ratio of the area under the curve for each signal now let's consider this example so we have the molecule one nitro propane and there's three different types of protons on it go ahead and match every proton or every different type of proton with the signals that we see here in the h nmr spectrum shown below feel free to pause the video and work out this example so the first thing we should do is identify the
different types of protons and we can clearly see that there's three of them proton a b and c now let's determine the multiplicity of each proton that we have in this molecule so let's look at the ch3 first proton a is adjacent to two other protons so using the n plus one rule we have two plus one which is three now proton c is adjacent to also to two protons so using the same m plus one rule two plus one is three now what about the one in the middle because right now we see that
we have two triplets corresponds to these two signals the one in the middle appears to be six or a sex tap and if we look at the adjacent protons we have three on the left two on the right so three plus two is five using the n plus one rule five plus one is six now this is basically a simplified version of the n plus one rule when you're looking at two different types of protons now it's important to understand that this is not the most accurate way to get the multiplicity for this type of
proton when there's two different types of protons adjacent to it in most cases it will suffice as you can see that proton b has to correspond to this one but it's not the best way it's not the most accurate way of doing it what we should do is this so analyzing proton b we need to look at the adjacent protons so proton a will split b using the n plus one rule so three plus one is four it's going to split it into a quartet so we have four next we need to look at the
other adjacent protons that is proton c and it's going to split protons b into a triplet because two plus one is three so what you're gonna have is four times three or three times four so really we should have twelve peaks for this signal however some of the peaks overlap and so it looks like as if it's six but it's really not it's four times three so that's how you're supposed to use the m plus one rule when you're dealing with adjacent protons that are not equivalent to each other so this could mean we have
a quartet of triplets or it could be three times four it can mean that we have a triplet of quartets i won't go into the specifics of that because that falls in the realm of complex splitting just know that when you're dealing with a proton in the middle if the adjacent protons are not equivalent you need to multiply the effect that they have using the n plus one rule so this is three plus one is four this is two plus one is three so you do four times three and should get twelve so instead of
calling this a sex tap which is what it appears to be here it's best to call it just a multiplayer and so far we have a triplet multiplet and a triplet so this has to correspond to proton b now which one is proton a and which one is proton c proton c is next to a very powerful electron withdrawn group the no2 group and so it's going to cause it to be downfield so it's going to have a high chemical shift so therefore this will be proton c between four and five proton a is up
field it's very far away from the electron withdrawing group so this will be proton a which is a triplet and so this corresponds to uh 2h let's see if i can put up here and this corresponds also to a 2h and this corresponds to a 3h so that's how you could use multiplicity to basically match up the protons with the signals that we see in the corresponding hmr spectrum now for those of you who may want to know what a quartet of triplets look like and a triplet of quartets here's how you can draw it
so let's start with a quartet of triplets so what this means is that we have four triplets so here's the first triplet with a one two one ratio and here's the second one and then here is the third triplet which is smaller and then the fourth triplet so we have four triplets and the ratio of the areas of those triplets will be in a one to three to three to one ratio which corresponds to a quartet so that is a quartet of triplets that's how it looks like now let's talk about a triplet of quartets
so this means we have three quartets so here is the first quartet let me draw a straighter line and here is the second quartet which is smaller than the one in the middle and here's the last one so those are the three quartets and each quartet has a ratio as you can see of one to three to one for their intensities however for each of the three quartets the ratio of the areas is going to be one to two to one which corresponds to a triplet so that's a triplet of quartets or three quartets now
let's talk about why we get the intensity ratios that we get for a doublet a triplet and a quartet so let's say we have this situation let's say we have proton a and proton b and we're analyzing the signal for proton a and there's only one adjacent proton to proton a as we can see here so using the n plus one rule this will give us a doublet and so i'm going to draw like this so let's see why the intensity ratio is one to one for a doublet now it has to do with the
way the protons are aligned with magnetic field every proton has its own magnetic moment it has its own magnetic field which can be aligned with the external magnetic field of the nmr spectrometer or it can be aligned against it so let's say this is the applied magnetic field proton b can have a magnetic field that is aligned with the applied magnetic field or it can have a magnetic field that's aligned against it now if it's aligned with the magnetic field then that effect on proton a will be a stronger magnetic field because those two lines
will add up and so this particular peak will appear downfield it's going to have a higher frequency due to the stronger net magnetic field now this peak appears with a lower frequency it appears slightly up field because the net magnetic field is weaker the applied magnetic field and the magnetic field of the adjacent proton they go against each other and so the net magnetic field is weaker and that's why it appears at a slightly lower frequency so because the magnetic field of proton b can have two possibilities either it can be aligned with the magnetic
field the external magnetic field or it can be aligned against it and these two possibilities are roughly equal that's why the intensity is in the one to one ratio but now let's look at another example so let's see if we have a triplet why the intensity ratio is one two one so we need another proton here so in this case proton a is being split by two protons two adjacent protons so two plus one will give us three we're going to get the triplet so let's draw a picture of the triplet to begin with so
it's going to look something like this so as we can see there's three peaks in this signal and let's draw the direction of the external applied magnetic field so we have two protons so we're going to draw an arrow for each of those protons so for this particular peak the protons have to be aligned with the magnetic field because it's furthest downfield it's at the highest frequency which means the net magnetic field has to be the strongest so both protons are in the same direction with that field now to get the one on the right
that peak corresponds to a low frequency so that's when the two protons will be against the applied magnetic field thus giving us the weakest net magnetic field that's acting on this proton so we have two fields that are aligned with magnetic field and two that are aligned against it now we could have one with and one against the magnetic field or the first one could be against it and the second one could be with it so thus we have our one two one ratio so this is when both protons are aligned with magnetic field and
this is when both protons that is these two are aligned against the magnetic field and in the middle you can have one that's aligned with it or one that's lying against it and so that's why we can see why we have a one to two to one ratio now let's consider the last case that is when we are going to get a quartet so we're going to need three adjacent protons and the intensity ratio is going to be 1 three three one so we're going to get something that looks like this so for the first
one all three of these protons will be aligned with the magnetic field i'm going to draw it here for the last one where it's up filled all three protons can have the magnetic field against the external magnetic field now for this one here we can have two that are aligned with the magnetic field and one will be against it so it can be up up down it can be up down up or it could be down up up so there's three possibilities in which two of the three magnetic fields will be aligned with the magnetic
field now for this one we're going to have one that is aligned with magnetic field and two against it so it can be up down down or it could be down up down or down down and then up so in this case we have all three that's aligned with the magnetic field here we have two aligned with the magnetic field and for this one there's only one that's aligned with magnetic field and for the last one there's none and you can see that we have a 1 to 3 to 3 to 1 ratio and so
now you know why we get the intensities that we see in the nmr spectrum and this is the basic idea behind spin spin splinter it has to do with the way that the adjacent protons align with the external magnetic field thus affecting the proton that we're analyzing
Related Videos
12:06
How To Draw The Proton NMR Spectrum of an ...
The Organic Chemistry Tutor
117,617 views
20:26
How To Determine The Number of Signals In ...
The Organic Chemistry Tutor
729,643 views
18:25
HNMR Splitting Patterns
Organic Chemistry with Victor
4,365 views
12:34
How to Interpret an IR Spectrum and Identi...
Melissa Maribel
10,360 views

19:27
NMR Spectroscopy: Compound Multiplets and ...
Danny Allwood
81,974 views

22:45
Beat Ronaldo, Win $1,000,000
MrBeast
107,629,821 views
15:26
Chemical Shift In NMR Spectroscopy
The Organic Chemistry Tutor
325,345 views
28:29
H NMR Spectroscopy Review - Examples & Mul...
The Organic Chemistry Tutor
539,926 views

8:43
This Was an Awful Idea
Daily Dose Of Internet
1,151,138 views
11:02
H-NMR Predicting Molecular Structure Using...
Leah4sci
343,598 views

15:33
IR Spectroscopy - Basic Introduction
The Organic Chemistry Tutor
761,673 views
11:49
Shielding and Deshielding - H NMR Spectro...
The Organic Chemistry Tutor
210,244 views
40:48
HNMR Practice Problems with Step-by-Step S...
Organic Chemistry with Victor
32,585 views

1:38:27
Carbon-13 NMR Spectroscopy
The Organic Chemistry Tutor
523,347 views

10:02
Mass Spectrometry
The Organic Chemistry Tutor
682,838 views
7:55
Complex splitting | Spectroscopy | Organic...
Khan Academy Organic Chemistry
342,145 views

14:36
NMR Spectroscopy
Professor Dave Explains
1,196,431 views
11:31
Proton NMR - How To Analyze The Peaks Of H...
Leah4sci
1,188,172 views
![H-NMR Spectroscopy Basics [Livestream Recording] Organic Chemistry Review & Practice Session](https://img.youtube.com/vi/zpBsAY8K89s/mqdefault.jpg)
58:07
H-NMR Spectroscopy Basics [Livestream Reco...
Leah4sci
23,353 views
5:29
Integration of H NMR Signals - Spectroscop...
The Organic Chemistry Tutor
237,831 views