First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
1.72M views1405 WordsCopy TextShare

The Organic Chemistry Tutor
This chemistry video tutorial provides a basic introduction into the first law of thermodynamics. I...
Video Transcript:
in this video we're going to talk about the first law of thermodynamics and how it relates to internal energy heat and work so what is the basic idea behind the first law of thermodynamics the gist of it is this energy cannot be created or destroyed it can simply be transferred from one place to another so let's say if we have a system and everything outside of it is the surroundings energy can flow into or out of the system and it's two ways in which energy can do so and that is through heat and work so
if heat flows into the system the system gains energy and that energy is known as the internal energy of the system represented by the symbol capital u now the surroundings can do work on a system so those are the two ways in which a system can increase its internal energy it's by the transfer of heat energy into the system or if the surroundings perform work on a system so let's say if the surroundings perform 300 joules on a system that means the system's internal energy goes up by 300 so the change is positive so delta
u increases the energy of the surroundings however has to decrease by 300 and so energy is not created or destroyed it simply transferred from one place to another the system didn't just get the 300 joules from nowhere that 300 joules of energy came from somewhere it came from the surroundings a good way to illustrate this is to use money so let's say if you decide to sell a laptop for five hundred dollars someone will buy the laptop for 500 let's say if someone does buy it your bank account will increase by 500 but that person's
bank account will decrease by 500 and so the money just doesn't come from nowhere just doesn't just magically appears it comes from somewhere so in order for you to gain 500 someone else has to lose 500. now granted the government could print more money if they wanted to but in a practical sense in everyday life when someone gains money someone has to lose money and so in that sense within that transaction money is not created or destroyed it simply transferred from one place to another and energy follows that same principle unless you're god energy cannot
be created or destroyed as far as we know but in today's world under practical conditions energy is simply transferred from one place to another so in our example if the system gains 300 joules of energy that means the surroundings loses 300 joules of energy if the system loses 500 joules of energy the surroundings have to gain 500 joules of energy and in that sense within that practical area energy is not created or destroyed thus we have the first law of thermodynamics now there's three types of systems that you want to be familiar with the first
one is an open system the second is a closed system and the third is an isolated system so in the open system matter can enter into an open system so oxygen from the air can go inside of it and also heat energy can flow into an open system so matter and energy can be transferred into and out of an open system now in a closed system matter cannot flow into it so oxygen just it can go inside of a closed system however energy can still flow into a closed system so in a closed system only
energy can go into and out of a closed system but matter cannot in an isolated system energy or matter cannot enter or leave an isolated system so the mass within an isolated system is fixed it doesn't change and the total energy of an isolate system also doesn't change because no energy can flow into it or out of it the equation for the change in the internal energy of a system is q plus w and this is the equation that you'll see in a typical chemistry textbook q represents the heat energy that flows into or out
of the system and w represents the work now in physics the equation is a bit different in the physics textbook you'll see delta u is equal to q minus w now you might be wondering why is it different why is it not the same and the reason for that is the point of view taken by the scientists in chemistry we take the system's point of view in physics engineers take the viewpoint of the surroundings so in chemistry w is negative when work is done by the system anytime work is done by the system the system
has to expand energy to do work and so the internal energy of the system decreases imagine if you're going to the gym to work out and as you lift weights your body is burning energy the internal energy of your body decreases as you burn calories and so work is being done by the system that is you're the system you're doing the work and the surroundings might be the waste that you're lifting so as you burn calories to lift up a weight from let's say the ground to an elevated position the potential energy of that weight
increases but the internal energy of your body decreases because you're burning calories you're losing weight in this case whenever w is negative work is done by the system in the case of chemistry and when w is positive work is done on a system now in physics it's different we take the surroundings point of view in physics so w is negative when work is done on the system and w is positive when work is done by the system so let's analyze these two cases when work is done by the system so in chemistry when work is
done by the system as we said before the internal energy decreases and because the energy of the system is decreasing work is negative remember we're taking the viewpoint of the system in physics we want to take the viewpoint of the surroundings so when work is done by the system the internal energy of the system still decreases however we say w is positive because the surroundings is gaining energy we're taking the viewpoint of the surroundings and so since the surroundings gain energy w is positive in the case of chemistry when work is done by the system
the system loses energy so we say w is negative and so it's the viewpoint and that's why the equations are a bit different in chemistry we take the viewpoint of the system but in physics we take the viewpoint on the surroundings so when work is done by the system the system loses energy the surroundings gain energy so if you're focused on the surroundings then w is positive because the energy of the surroundings go up if you focus on a system the system loses energy when work is done by the system and so w is negative
but in this video i'm going to take the system's perspective so going back to this equation delta u is q plus w you need to know that q is positive whenever the system meaning the reactants and the products absorb heat energy so anytime heat flows into the system heat energy is absorbed by the system so q is positive this is an endothermic process whenever heat energy is absorbed now q is negative whenever the system releases heat energy and so heat energy flows out of the system into the surroundings and so this is an exothermic process
so anytime heat energy is released it's exothermic and when heat energy is absorbed by the system it's endothermic so for an exothermic process q is negative and for an endothermic process q is positive and as was mentioned before four w is positive when work is done on the system this is in chemistry and w is negative whenever work is done by the system so you need to be familiar with the sign conventions if you're gonna use this equation so in another video i'm gonna give you some practice problems on calculating the change in internal energy
using heat and work you
Related Videos
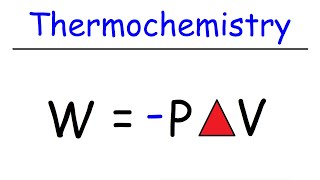
29:27
Thermochemistry Equations and Formulas Wit...
The Organic Chemistry Tutor
158,309 views
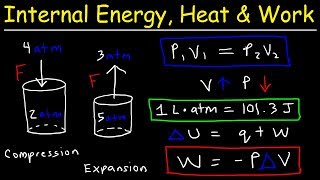
23:29
Internal Energy, Heat, and Work Thermody...
The Organic Chemistry Tutor
478,249 views
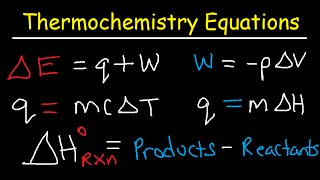
21:17
Thermochemistry Equations & Formulas - Lec...
The Organic Chemistry Tutor
1,421,367 views
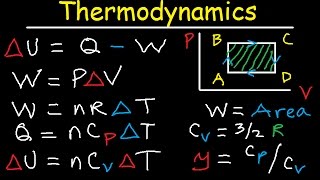
3:05:16
Thermodynamics, PV Diagrams, Internal Ener...
The Organic Chemistry Tutor
2,439,535 views

6:11
The physics of entropy and the origin of l...
Big Think
860,589 views

17:40
First law of thermodynamics / internal ene...
Khan Academy
1,523,656 views
17:15
All physics explained in 15 minutes (worth...
Arvin Ash
5,160,654 views
8:12
The Laws of Thermodynamics, Entropy, and G...
Professor Dave Explains
2,612,931 views

27:11
You're Probably Wrong About Rainbows
Veritasium
2,802,502 views
19:24
Gas Law Formulas and Equations - College C...
The Organic Chemistry Tutor
182,417 views

27:15
The Most Misunderstood Concept in Physics
Veritasium
17,010,858 views
29:59
5.1 First Law of Thermodynamics and Enthal...
Chad's Prep
30,730 views

10:04
Thermodynamics: Crash Course Physics #23
CrashCourse
1,772,245 views

14:03
Hess's Law Problems & Enthalpy Change - Ch...
The Organic Chemistry Tutor
1,222,986 views

52:48
Lecture 1: Introduction to Thermodynamics
MIT OpenCourseWare
88,708 views

32:44
The Strange Physics Principle That Shapes ...
Veritasium
6,718,152 views

1:18:26
Carnot Heat Engines, Efficiency, Refrigera...
The Organic Chemistry Tutor
419,995 views
27:37
Calorimetry Problems, Thermochemistry Prac...
The Organic Chemistry Tutor
1,213,476 views

46:46
Lec 1 | MIT 5.60 Thermodynamics & Kinetics...
MIT OpenCourseWare
1,592,615 views