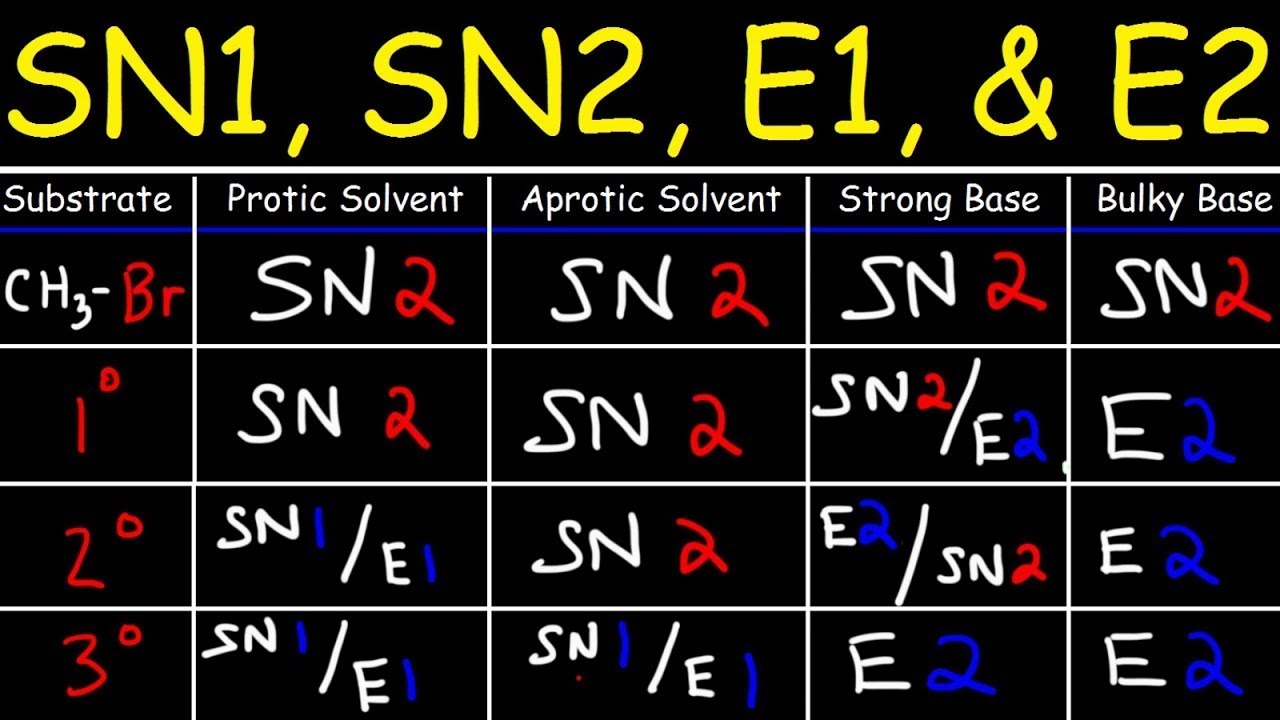
hey guys, professor, Dave here. I know you're probably having a hard time with your SN2, SN1 E2, E1 mechanisms, especially deciding which one is going to happen well don't worry, I'm here with some basic tips that will help you decide when we're learning these first few mechanisms a very common substrate is a simple haloalkane so we want to understand that the different substitutions that are possible, or degrees of substitution of each haloalkane are gonna tell us which mechanisms can or cannot happen so for example we look at this tertiary haloalkane, we have to understand that SN2 is not going to be possible because there is simply too much steric hindrance for a nucleophile to approach that tertiary center so steric hindrance means there's a lot of alkyl groups, that a lot of electron density that is going to repel any incoming nucleophile that is negatively charged so with a tertiary haloalkane we can rule out SN2 that's not going to occur, however SN1 is a very good candidate because we know that the more substituted that a carbocation is the more stable it is due to the hyperconjugation from the neighboring alkyl groups so if this bromine were to leave it would leave a tertiary carbocation which is stable so SN1 is a very good candidate therefore E1 is also good candidate and E2 is also possible. so basically with tertiary me know no SN2.
with a primary haloalkane SN2 now is very possible, there's not very much steric hindrance so it's going to be very possible for a nucleophile to approach this carbon in this direction and so SN2 is going to be possible, E2 is also possible however SN1 and E1 are very unlikely to happen because if this a bromine is to leave that would leave a primary carbocation a primary carbocation is very unstable because it does not get to enjoy very much hyperconjugation from neighboring alkyl groups so because the primary carbocation is very unlikely to form we can more or less rule out E1 and SN1 for a primary haloalkane there are some exceptions like an allylic system but at this stage we can pretty much just take it for granted that a primary carbocation is very unlikely to form. with a secondary haloalkane there's not that much information that we can get if there's a lot of beta branching or some steric hindrance more towards the sides that might be a factor in repelling a nucleophile that's going to do SN2 but basically with a secondary haloalkane we can't really gather that much information about what mechanism is going to occur just by looking at the substrate, but for tertiary and primary we are able to rule out some possibilities the next thing we want to look at is the strength of the nucleophile and leaving group so the first thing we want to understand is that nucleophilicity parallels basicity, this means that there's a correlation between how much a group is a able to coordinate to a proton and how well group is able to coordinate to a molecule, so something that's a very strong base seeks to form a bond to a proton but it could just as well act as a nucleophile and and coordinate to a molecule so for example hydroxide is a much stronger base than water, it has a formal negative charge, it's a strong base. a water molecule is not very basic, in fact it's neutral.
so that means that if let's say we keep it real simple, we're just trying to decide between SN1 and SN2, we'll take a look at the eliminations later if we look at this substrate and we look at what would happen whether hydroxide or water were to attack now the hydroxide is a very strong base so it's a very strong nucleophile so it is capable of an SN2 reaction, so there's the SN2 product however a water molecule is not strong enough to do SN2, it's not a strong nucleophile so but what could happen is that if the bromine leaves, leaving a carbocation intermediate that is a very unstable situation so water molecule would be a strong enough nucleophile to coordinate to a carbocation and then deprotonate so basically a water molecule is capable of behaving as a nucleophile in an SN1 reaction. recall that if we do an SN1 we're gonna get a racemic mixture, so there's the two enantiomers so that's the main takeaway, we want understand that nucleophilicity parallels basicity. now that the halides are going to be very common nucleophiles as well as leaving groups so we want to understand how those behave in a polar aprotic solvent, meaning the solvent does not have any protons that are available for dipole-dipole interactions it is going to be the case that the that fluorine or I should say fluoride, let's make these all the anions the fluoride is going to be the strongest nucleophile because it is the strongest base of the halide ions but the reason for that is that as you go down the periodic table, you go down the halogens the ionic radius is getting larger as we go and if we have a larger ion then that becomes more polarizable so what that means is that its a larger volume with which that ion can diffuse and spread apart, spread out its negative charge so because the negative charge on the fluoride is highly localized on a very small ion, that makes it a very strong nucleophile all the halide ions are good nucleophiles but in a polar aprotic solvent it's going to be fluoride that's the best.
now if you're looking at a polar protic solvent this trend is gonna reverse because by the same token if the fluoride is much more basic that means that it is gonna spend the most time in solvent interacting with any available protons let's say the protons on a water molecule, so we can see here water is a polar protic solvent because these protons are available for ion-dipole interactions, so this is an ion-dipole interaction and there are several that the fluoride is able to participate in because it has a very localized charge so because the fluoride ion is spending much more time interacting with molecules of solvent that is going to hinder its ability to act as a nucleophile, whereas is it still will be able to but in this case the iodide is going to be the best because its charge is more spread out, more diffuse, it's more polarizable so it is going to be spending less time participating in interactions with molecules of solvent which will make it the best nucleophile of those. so leaving group ability is basically the opposite side of the coin so its precisely for the same reason that hydroxide is a very very strong nucleophile that's the same reason why it's a terrible leaving group so hydroxide is very basic, it's unstable it seeks to coordinate to something so it should follow that if it's such a good nucleophile then it's not really going to be in a rush to leave so that's why a hydroxyl group is going to be a bad leaving group because it's so basic. however if we tack on another proton another proton makes that a water molecule water is a very good leaving group because a water molecule will be very stable once it has left so that's why the halides as well are very good leaving groups because bromide is perfectly happy on it's own, it's got a full octet so that's not gonna be a problem and we can look at the same rules with which we examined the nucleophilicity of the halides to examine which ones are going to be the best leaving group because we can see depending on whether it's a polar protic or aprotic solvent we will know how well it is going to interact with the protons in solvent but those are all going to the pretty good leaving groups.
a third thing that we want to look at is the steric hindrance associated with the nucleophile so let's take a look at these, the hydroxide and some alkoxides so these all have O minus as they're trying to behave as a nucleophile, so we have roughly the same basicity. there's a small variation but it's all O minus so there's not much difference in the basicity here so technically the nucleophilic strength is roughly the same however look at what's happening as we are adding more and more carbons branching this is increasing the steric hindrance in the nucleophile and there is a point where a nucleophile will no longer be able to approach a substrate because of all the steric hindrance and so hydroxide being very small is able to approach even a secondary system with the beta branching, itdoesn't really matter it's quite small however a tert-butoxide is the classic E2 promoter because it is simply to sterically hindered to approach a substrate and substitute so the more sterically hindered the base the less likely it is to substitute and the more likely it is to eliminate. the last thing we want to look at his temperature so let's take a system like this, we've got a secondary haloalkane, we've got hydroxide.
hydroxide is very strong, you're probably looking at SN2 or E2. we can look at temperature to choose which one is going to occur so let's take two hypothetical temperatures, let's call 0 a cold temperature and 100 is gonna be a hot temperature so it as it turns out cold temperatures favor substitution so at 0 it's much more likely to get the SN2 product, and hot temperatures favor elimination so at 100 we're much more likely to get one of the two possible elimination products. now the reason for this lies in the Gibbs free energy equation.
I hope we will remember this from gen-chem. so Delta G equals Delta H minus T Delta S so Delta G is the term that is Gibbs free energy and refers to the spontaneity of any given reaction so a negative value for Delta G means it's going to be a spontaneous reaction, positive will be non-spontaneous Delta H is a change in enthalpy so that's basically energy, the energy that's associated with the with a change in the reaction and then T is temperature. Delta S is a change in entropy or disorder so one thing we need to understand is that an elimination reaction is more entropically favorable than a substitution reaction, and here's why.
we have a substrate and we have a nucleophile, we have the product and the leaving group so that's two molecules or atoms generating two things, so two things two things. however with an elimination so we have a the same set up at the start here but look what happens at the end, we have the product ,we have a water molecule and a bromide, so two things become three things more disorder, that's entropically favorable. now the thing about the equation is that the change in entropy is being multiplied by temperature, so that means that at very high temperatures the entropic favorability or unfavorability becomes a greater factor in determining the spontaneity of the reaction so let's say we have a very entropically favorable situation like an elimination at a high temperature this whole T Delta S term becomes more significant and it becomes more likely that Delta G is gonna end up having a negative value so that's why at high temperatures elimination is going to be favored it can be tricky to choose which mechanism is occurring but as long as we look at the substrate and the nucleophile we ought to be able to eliminate some options so let's put it all together and look at some examples if we're gonna look at something like this the first thing we can do is look at the substrate and see if we can eliminate any of the possible mechanisms so this is a primary haloalkane so immediately we can get rid of SN1 and E1, we're not going to form that primary carbocation and SN1 and E1 both require a carbocation intermediate to occur so we're gonna get rid of those options, we know it's going to be a SN2 or E2.
likewise if we look at the strength at the base, that's a strong base a strong nucleophile, it doesn't have to wait for a carbocation to occur it can bully around the substrate and make a reaction happen so by that reasoning again we're looking at SN2 or E2, so we've just gotten rid of half of the options and now we're down to SN2 and E2 just from looking at the substrate and the base so between SN2 and E2 we might have to look at maybe a temperature. let's say we're doing this at a hundred degrees that is going to cause us to favor E2 because hot temperatures favor elimination so number one we're gonna go with that as our E2 product. so number two same setup right, but both by looking at the substrate and the base both those things are telling us that we're looking at SN2 or E2 again now looking at the cold temperature this time maybe we're gonna favor SN2, so number 2 we're looking at that as our most likely product SN2.
so again we're looking at the substrate, we're looking at the strength of the base and we're looking at a temperature, that's gonna allow us to figure out what's going on. so let's look at this one now, we have a secondary substrate so with the secondary haloalkane we're not really gonna be able to figure out too much about which reaction or which mechanism is going to occur but we're looking at water as the nucleophile so water is a weak base so it is a poor nucleophile, that means we can get rid of SN2 and E2 as possibilities because it's not a strong enough nucleophile to do either of those reactions, either of those mechanisms, so we know we're gonna get SN1 or E1. between the two we can look at the temperature again this time let's say zero so that means we're gonna favor SN1 because colder temperatures favor substitution so we know that we're gonna be looking at this and that is our SN1 product.
okay so once again we're just looking at the base and we're seeing that that is weak that is a weak nucleophile and a weak nucleophile will not be able to do SN2 or E2 so we're getting rid of those possibilities and then we look to the temperature. so how about this, we've got the same substrate now we've got that tert-butoxide so if we remember from before tert-butoxide is the classic E2 promoter. now the reason for this is it is strong, it is an alkoxide, it has a formal negative charge, so alkoxides are strong bases so a strong base is a strong nucleophile so it's going to be able to do SN2 or E2 it does not need to wait for a carbocation it's gonna make something happen, it sees the substrate and it is either gonna try to coordinate or is gonna do an acid-base reaction.
because it's a strong base now the thing is that, remember before we were talking about steric hindrance of the nucleophile, there is too much steric hindrance associated with this nucleophile for it to coordinate it cannot do SN2 so this can only do E2 so there is our E2 product so remember, tert-butoxide always gonna go E2. so now let's look at a tertiary haloalkane. so with a tertiary we can remember we're not going to be able to do SN2 so we're we're getting rid of that possibility the thing is hydroxide is strong, hydroxide is a strong base so it's a strong nucleophile so it's able to do SN2, E2, it doesn't need to wait for a carbocation to form so SN1 and E1 aren't going to happen, that's just going to take too long hydroxide finds a substrate in solution it's gonna bully it around, it's going to do something but we just said it can't do SN2 so the only possibility is therefore E2, we don't even have to look to a temperature we're seeing that this is gonna want to either substitute or eliminate it cannot substitute because of the steric hindrance associated with the substrate this time so that is going to give us the E2 product so number five, we added a carbon this time so there's that so there is our E2 product incapable of doing SN2.
now same substrate now we're looking at methanol, so methanol is weak, there is no formal charge there it is comparable to water in terms of basicity, just has that extra methyl group instead of a hydrogen but it's completely covalent, there's no its neutral, there's no negative charge so that is a weak base so it's a poor nucleophile so it is not strong enough to you SN2 or E2 so it's gonna be SN1 or E1, we're going to look at the temperature 100 degrees, once again that favors elimination so but it is weak so it's gonna do E1, so it has to wait for that bromine to leave, once that bromine leaves and we have a carbocation then those beta protons become much much more acidic such that even a weak base like methanol is going to be able to extract one. so let's say number six is our E1 product so here's a few examples and and we're able to see how each mechanism could be promoted in certain situations depending on the substitution of the substrate strength of the base or nucleophile and the temperature, so as long as you just think critically about any example you're given you can always look to something like this and begin to get rid of something, whatever's going on and narrow it down to the answer. thanks for watching, guys.