Southern Blot Method - Animated Video
219.97k views1152 WordsCopy TextShare
Biology with Animations
I make animations in biology with PowerPoint, and this animation video is about southern blot method...
Video Transcript:
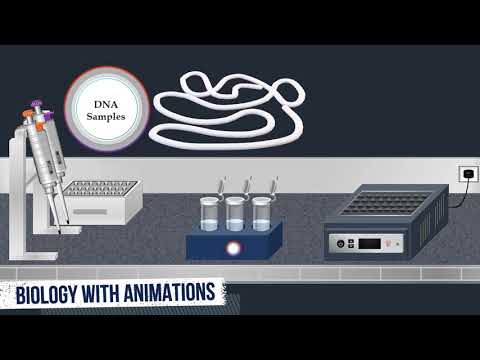
southern blood is a classic technique in molecular biology that reveals information about dna identity size and abundance and it can be used for detection of a specific dna sequence in dna samples the first step in southern blotting is the digestion of the dna samples with an appropriate restriction enzyme a restriction enzyme also called restriction endonuclease is an enzyme that cleaves dna into fragments at or near specific recognition sites within molecules known as restriction sites once the restriction enzyme is added the samples are incubated at 37 degrees celsius overnight restriction enzymes recognize a specific sequence of
nucleotides and produce a double-stranded cut in the dna therefore various dna fragments of different sizes are obtained then the dna fragments produced by restriction endonuclease digestion are separated by gel electrophoresis for gel electrophoresis separation a loading buffer is added to the dna samples and it is used as a tracking die which migrates in the same direction as dna allowing the user to monitor the progress of the separation agaris gel electrophoresis is most commonly used to separate mixtures of dna fragments of varying sizes during electrophoresis a molecular weight size marker known as a dna ladder is
commonly used to determine the size of dna fragments in the samples the dna ladder is added into well at one end of the gel the molecular weight size marker is added the dna samples are loaded into wells then an electric current is applied to pull the samples through the gel based on their charge and size the molecules will travel through the gel at different speeds allowing them to be separated from one another the phosphate backbone of the dna molecule is negatively charged therefore when placed in an electric field dna fragments will migrate to the positively
charged anoda because all dna fragments have the same amount of charge per mass small fragments move through the gel faster than large ones after the electrophoresis is complete the molecules in the gel can be stained to make them visible when the gel is stained with an intercalating dye such as ethidium bromide the dna fragments can be seen under uv light as bands each representing a group of same size dna fragments after the separation of the dna molecules the double-stranded dna fragments are denatured with an alkaline solution consisting of sodium hydroxide the gel is soaked in
the alkaline solution with gentle agitation at an alkaline ph hydroxide ions are predominant consequently guanine and thymine will be deprotonated and exist as negatively charged conjugate bases this process breaks the hydrogen bonds between the oligonucleotides causing the separation of the two dna strands after the denaturation treatment the alkaline solution is removed then a neutralizing solution is used to neutralize the ph of the gel this allows for a more efficient transfer of dna in southern transfers while the gel is neutralizing filter paper and membrane are prepared for southern blotting to transfer the dna fragments to the
membrane a transfer buffer a solid support and a sheet of blotting paper acts as a wick for the transfer solution are used the wick is placed over the solid support in the transfer reservoir so the ends will be in the transfer buffer then it is wetted with the transfer solution next pieces of extra thick blotting paper are placed on top of the wick then they are wetted with the transfer solution once the gel is neutralized it is placed on the thoroughly wetted wicking paper then a sheet of nylon or nitrocellulose membrane with the same size
as the gel is pre-wetted with the transfer solution and placed on top of the gel next pre-wetted pieces of extra thick blotting paper are placed on top of the membrane the exposed areas of the wick are covered with strips of plastic wrap to prevent transfer buffer from bypassing the gel during the transfer process finally a dry stack of paper towels is placed on top of the membrane and gel then a glass plate is placed on top of these sheets paper with a weight to maintain tight contact between the gel and membrane buffer transfer by capillary
action from a region of high water potential to a region of low water potential is then used to move the dna from the gel onto the membrane consequently ion exchange interactions bind the dna to the membrane due to the negative charge of the dna and positive charge of the membrane the transfer is allowed to proceed overnight then once it is completed the blotting material and membrane are carefully removed from the gel next the membrane is briefly rinsed to remove any agaris that may be stuck during the transfer then it is exposed to ultraviolet radiation to
permanently attach the transfer dna to the membrane after attachment of the dna fragments to the membrane hybridization with radio-label dna probes is performed the membrane is placed in a bottle containing a pre-hybridization solution which is used to reduce non-specific hybridization with the probe next the bottle is incubated in hybridization oven at 42 degrees celsius for two hours once the incubation is complete the pre-hybridization solution is removed then a hybridization buffer is added into the bottle next labeled dna probes are added to the hybridization solution the probes are fragments of dna of variable length which can
be radioactively or fluorescently labeled once the hybridization probes are added the bottle is incubated overnight in the hybridization oven at 42 degrees celsius dna contains a large quantity of phosphorus in the phosphodiester linkages between bases in the oligonucleotide chain dna can therefore be tracked by replacing its non-radioactive phosphorus with radioactive phosphorus 32 the radioactively labeled dna probes hybridized to their complementary sequences in the dna fragments after the hybridization of each probe to its target sequence the hybridization solution is removed next a wash buffer is added into the bottle then the membrane is incubated at 52
degrees celsius for 30 minutes the washing process is repeated 3 times to remove unbound and weekly binding probe after hybridization an autoradiography method is carried out to identify the location of radioactively labeled dna in the membrane the southern blot filter is placed inside a light proof cassette box then an x-ray film is laid over the top the cassette is closed and left for several hours to several days the radio ice at a playable dna exposes the film which when developed shows a pattern of black bands that indicate the positions of labeled dna in the blot
membrane subsequently these informations can be used to determine which gene is present in each sample also the position of each gene can be identified in the gel consequently after electrophoresis bands of interest can be cut out from the gel so that each gene can be isolated for further analysis you
Related Videos

11:46
Western Blot Method - Animated Video
Biology with Animations
139,845 views

8:11
Northern Blot Method - Animated Video
Biology with Animations
65,365 views

7:55
Gel Electrophoresis
Amoeba Sisters
2,395,891 views

8:20
Coronavirus Test: Real time RT-PCR - Anima...
Biology with Animations
5,542,654 views

8:38
SDS-PAGE, Sodium Dodecyl Sulfate–PolyAcryl...
Biology with Animations
564,457 views
5:10
Southern blot | Biomolecules | MCAT | Khan...
khanacademymedicine
410,162 views

14:27
How San Francisco is Preparing for a Mega-...
The B1M
27,321 views

6:26
Southern Blot
Abnova
178,682 views
4:15
Southern Blot
Quick Biochemistry Basics
181,394 views

13:16
Recombinant DNA Technology - Animated Video
Biology with Animations
58,844 views

5:27
Agarose Gel Electrophoresis - Animated Video
Biology with Animations
152,661 views
16:39
Western blot explained in details | Applic...
Animated biology With arpan
21,868 views

7:20
DNA animation (2002-2014) by Drew Berry an...
WEHImovies
6,027,314 views

7:09
What is PCR and qPCR? | PCR Animation
ClevaLab
200,016 views

37:26
Molecular animation – Tech Talk by Drew Be...
WEHImovies
82,793 views

8:08
Southern Blotting
Frank Lectures
204,394 views

4:23
Western Blot / Protein Immunoblot explained
Henrik's Lab
337,501 views

3:56
PCR - Polymerase Chain Reaction (IQOG-CSIC)
CanalDivulgación
4,489,918 views

10:56
Western Blotting (Immunoblotting) : Princi...
Frank Lectures
373,989 views
6:36
Maxam–Gilbert DNA Sequencing Method Animation
Biology with Animations
227,485 views