Ron Vale (UCSF, HHMI) 1: Molecular Motor Proteins
877.62k views5649 WordsCopy TextShare
Science Communication Lab
https://www.ibiology.org/cell-biology/motor-proteins/
Molecular motor proteins are fascinating enzy...
Video Transcript:
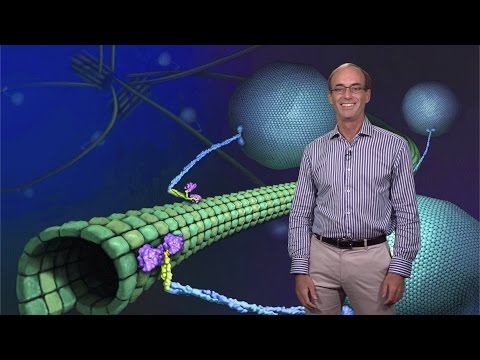
hello I'm Ron Veil and in this talk I'd like to introduce you to molecular motor proteins which are these fascinating protein machines that are featured in this animated video here and these proteins are able to walk along a cyto skill track and transport a variety of different types of caros inside of cells what I'd like to do today is first of all tell you a little bit of a history of biological motility and then I'll tell you about what these motor proteins do inside of cells the various functions that they have and then I'll tell
you a little bit about how they work how they're able to convert chemical energy into motion and finally I'll tell you uh some things about how these molecular motor proteins are relevant to human health uh and disease so first of all biological motion is just a fundamental and very obvious attribute of all living organisms for example terms of people were're able to run and uh a variety of animals are able to move and explore their environment and this is due to the fact that we have muscles uh and they can contract and uh theories about
how muscle work in fact very old uh dating back to the ancient Greeks A Whole New World of life was evident uh with the invention of the microscope and with that also a whole new world of motility um Leen hook for example when he looked at pond water under the microscope saw a whole variety of different types of movements and this is what he had to say about them the motion of most of them in the water was so Swift and so various upwards and downwards and roundabout that I admit that I could not but
Wonder at it indeed this uh all these types of biological emotions are indeed very fascinating um to Leen hook and also uh fascinating even to school kids today um now not only do cells move but material inside of cells can also undergo motion and the first person that described this was uh bav ventur Cy where he described cytoplasmic streaming inside a cells um and this is shown here in this video and what he described is uh shown here I know that the phenomena that I announce is too strange to be believed at first I saw
two torrents inside each section one of the torrent rose on one side and descended on the other constantly and this not once but thousands of times for days and for entire weeks you can see that these early scientists were were uh quite animated in their use of language to describe their scientific results something that I think uh scientists have lost today um now mic microscopy continued to play a major role in our understanding of biological motility and uh a Pioneer in this was Shin inaway uh who used more advanced types of microscopy uh such as
polarization microscopy to observe the Dynamics of living cells and he did a lot to describe uh the process of of cell division which is shown here where you can see the mitotic spindle you can see the motion of the mitotic spindle and also the movement of chromosomes here uh in this beautiful movie now a real the next Revelation I would say revolution occurred uh with the development of fluorescent proteins and now we can take that same object that you saw that chinya inaway described uh where he had to observe uh the mitotic spindle with natural
contrast uh from the polarization microscope but now we can tag specific proteins in the cell uh shown here are microtubules which are tagged in red um uh with a red fluorescent protein and the chromosomes with a green fluorescent protein uh and if you uh look at this in a dropa embryo you can see uh the chromosomes the formation of the of the mitotic spindle and uh the physical motion of these uh chromosomes here and when people began to tag a lot of proteins in the cell they found that they were uh not just static but
in fact in motion and now we know that a whole variety of molecules inside of cells are actually undergoing active uh types of movement to be localized uh in specific destinations in the cell now all of these types of biological movements that I I've described are due to the actions of these special enzymes called molecular motor protein which interact with cyosc tracts and there are two main types of cytos tracts one are the larger diameter uh microtubules and the smaller dynamer uh actin filaments uh and these are the two major cyosc filaments that act as
tracks for these molecular Motors so in the microt tual world there are two main types of soscal of Motors one are the dining molecules and I'm going to talk much more about these uh in my next two I biology lectures and kin which will be a focus of this lecture they move along microt tibial tracks which are shown in this movie over here and you can see that these microtubules extend all throughout the cell um and they're also themselves uh quite dynamic they can grow and Shrink uh and and change their position in cells as
well now these tracks both microtubules and actin are polar filaments and the reason is that they're composed of a basic uh subunit in this case uh tubulin which polymerizes in a defined head to tail manner to create this polymer so uh because the polymerization uh is polarized there's a distinct plus end of the microt tual and a distinct minus end and the motors recognize this intrinsic polarity of the tracks so for example the majority of kesin will move in one direction towards the plus end of the microt tual whereas dings move in the opposite direction
uh towards the minus end now these microtubules and cells are organized also not randomly but with a specific polarity so many of the microtubules are nucleated and grow from this structure by the nucleus which is called the centone and this is where the minus ends of the microt are located and they extend outward to the periphery where the plus ends are Lo localized so if a kinan motor grabs hold of a cargo it's going to move uh towards the plus end and it's going to deliver that cargo from the interior of the cell to the
periphery dining on the other hand will move in the opposite direction so it will deliver uh cargo uh towards the cell interior now the other major class of molecular Motors uh work on actin filaments and there's one major class of these motors and those are the mein Motors um now they move along actin which tends to have a a very different distribution than microtubules um so in this mitotic spindle for example the microtubules are in the center and you can see the actin in red all around the perimeter of the cell so actin in general
tends to be more cortical the microtubules more interior now when I say mein or tenin or dining I'm not just talking about one uh molecular motor in fact these are families of related uh motor proteins in humans for example there are 45 different uh kinesin genes there are 40 mein and about 15 Dings And there are so many different types of Motors because there are a whole variety of motility functions that are needed uh uh in human cells and these different Motors carry out different types of transport or Force generating functions now I'd like to
tell you a little bit about the anatomy of these motor proteins what makes motor proteins in the same family similar and also how they differ so they have one end of the motor protein which uh is the actual uh part that moves along the track and another part that interacts with the cargo and I'll show you this in more detail for uh one member of the kinan family called kinan one uh and uh this globular domain at the end I just showed you is the motor domain that's the part that walks along the track many
of these motor domains all also come together as diers in some cases even tetramers and that dimerization is um mediated by a coiled coil uh which is also interrupted uh it has hinges in it and that provides flexibility for the motor protein to bend and at the very distend is the tail domain so this may contain uh a globular domain belonging to the motor polypeptide it may also bind other Associated subunits uh to make a lar larger complex and this tail domain is what defines uh usually what that motor protein will bind to in the
cell what kind of cargo it will transport now uh there are other classes of motor proteins this is shows uh kinesin 2 and kinesin 3 and all of these motor proteins uh share in common a very similar uh motor domain um which is shown here but the rest of the protein is really completely different so the coil coils are different and the cargo binding domains in fact have no sequence identity uh between them and the reason is that these uh non-motor domains again are defining unique types of caros that these motors are interacting with in
inside of cells so let me tell you a little bit about the motor domain and what it does so it's an enzyme and it's an enzyme that hydes ATP so it first uh binds an ATP molecule Denine triphosphate and then it hydes uh a bond between the beta and gamma phosphate uh and after the hydrolisis it then releases the products in a sequential manner so it first releases a phosphate um then next the ADP is released uh and then it's able to rebind uh ATP and start the cycle all over again and during one round
of this ATP hydrolysis cycle there's structural changes occur in the motor that I'll describe later that allow it to take one step along the track so every time it undergoes this cycle it steps forward uh and then multiple rounds of this ATP hydrolysis uh cycle allow the motor to move a long distance along the track now let me just say a few things about uh how this motor performs in comparison to um a motor that you might be more familiar with like a car engine so first of all obviously uh the kesa motor is much
smaller in fact that motor domain is only 10 nanometers in size um it uses a fuel as I told you in the last slide uh adenosine triphosphate uh comparison to your car which uses hydrocarbons it moves at a few millimeters per hour which seems uh very slow uh however you have to take in account the size of the motor protein and if you uh uh measure how far it moves in terms of its own length uh you find in fact that it's moving uh faster than your car is moving on a highway it's also much
better than your car in terms of work efficiency uh which is essentially how much of the chemical energy that it can convert uh into productive work and these motor proteins work at about 60% efficiency whereas your car engine uh works at a much more pathetic 10 to 15% so we indeed have a lot to learn about how uh Nature's Own Motors are able to uh execute motility so let me tell you now a little bit about what these cytoskel motors do in cells and I'm just going to provide a few examples because the number of
different types of motility uh that cells uh engage in is really vast so one thing that the motor proteins do is to transport uh membrane organel uh in some cases they're small organel they're transport intermediates that are traveling between the G apparatus and the plasma membrane or endosomes that are traveling in the opposite direction from the plasma membrane to other organel such as lysosomes and this just shows an example of in living cells uh some of these transport vesicles moving along microt tual tracts but even very large organel also can move in cells probably the
biggest organel of all uh is the nucleus and this just shows an image of the nucleus uh being transported inside of a nerve cell uh which during development is migrating uh towards the cortical region uh of the brain and it has to transport the nucleus uh during this migration process um now here's another uh beautiful example of organel movement uh these are mea pigmented organel called melanosomes that are found in a special skin cell called uh melanocytes and this is what gives skin its color and some organisms such as uh amphibians and also fish can
change the color of their skin and they do that by moving uh these melanosomes when the melanosomes are dispersed like this the skin color appears darker uh but when they're all concentrated in the center the skin be takes on a lighter color and this distribution of organel occurs by Motors here are dining Motors transporting all these melanosomes towards the cell center and uh kinan Motors can transport these organel in the in the opposite direction and this is under hormonal control so hormones interact with receptors that control these motors to change the distribution inside of cells
but also there are other objects that are not membrane bound that also can be transported for example there are many kinds of viruses that have learned to pick up molecular Motors and transport themselves inside of cells this is an example of vaccinia virus and all these little particles these Myriad of little particles that you see here are viruses moving along microt tibial tracts another example being rabies which can transport itself self inside of uh nerve cells now in addition uh mrnas also can be localized and transport in cells and that allows the MRNA to be
localized in a particular region of the cell where that mRNA can be translated into a protein and therefore the protein is made where the protein is needed in a localized destination and this is an example of uh one RNA called girkin that is transported in a dropla oite it's shown here in this orange color and you can see it moving into this one uh anterior corner of the oite uh by active transport now in addition to transporting cargo Motors can move the filaments themselves and this is how a muscle contraction works and the basic unit
of a muscle is called a sarum and it's a repeating unit of actin and mein in the sarir mein is concentrated uh in a filamentous form in the center of the saram and it interacts with interdigitating uh actin filaments which are coming in from both sides of the sarir so masas uh wants to walk uh uh in One Direction on this side towards the end of the actin and the other side of the me filament is trying to walk in this direction along the ACT so when a muscle is activated which occurs with nerve stimulation
calcium comes in that's what calcium is shown here and the myin starts walking and it starts bringing these actin filaments uh closer together that shortens the sarcomere and that's what causes your muscle to contract in this case the filaments and the motors are very well organized in your muscle but Motors can also take a disordered a almost random array of filaments and create order uh amongst these filaments and this is what happens in the formation of the myotic spindle so in this case the microt tual start off random but then there's some Motors uh such
as shown in green here that are crossbridging filaments and they're trying to move to the minus end so they're moving to the minus end and as they do that they collect all the minus ends of the microt tual together there are other Motors such as shown in Orange that work on anti-parallel uh filaments and they work to slide those filaments apart so in this manner uh as shown in this movie of uh formation of a my myotic spindle in a zenopus egg extract you can see that this initial random uh organization of microtubules uh as
it grows the motors act upon it and they start elongating the spindle um and the minus ends all get organized at this pole and it forms this characteristic bipol polar shape due to the action of these molecular Motors so um I now like to turn to the subject of how do we study the mechanism of motility how do we uh understand how these motors work well um it's useful to look at the motility in cells but it's more powerful uh to study these Mo Motors in a test tube where you really can be able to
dissect their mechanism so uh we're able to use invitro motility assays where we can take either purify Motors out of cells or even express them uh in bacteria and study their motility in a very controlled environment in fact one can also do that at single molecule level so one can study the actions of even individual motor proteins uh as they are being transported on a filament so I'll show you some examples first of invitro motility assays here's an example where we have uh a plastic bead uh to which one can attach a motor Pro prot
uh and then the motor will transport these beads along a filament this is shown here in this movie here these are inert uh plastic beads being transported by Canan along a microt tual track now we can in fact get rid of the whole bead uh entirely uh and label the motor protein with a fluorescent Dy now this is not really uh drawn to scale here in fact the D is very small relative to the motor uh but it provides a very bright signal that we can track the motor protein as it's moving and we can
do that with a technique called total internal uh reflection fluorescence which is also described in uh videos in the I biology microscopy course but this is what it looks like all of these Green Dots here are individual motor proteins and you can see them uh being moved along these individual along these microtubules here so you can track and follow the details of this motion at a single molecule level one other type of invitro motility assay is one where the motor proteins are all coating a glass surface and in this case the motor proteins can't move
but they grab hold of the filament and then they start transporting this filament uh along the track and you'll see this in this video here these are microtubules and you can see them all sliding across the uh glass surface driven by these molecular motor proteins Now using this uh uh these types of invitro assay you can actually measure a lot of uh details of how these motors work for example using an optical trap again described in the ibiology microscopy course you can measure the forces produced by individual Motors uh the optical trap grabs hold of
a bead and tries to keep the bead in place on the other hand the motor is trying to move along the track uh and trying to move the bead out of the optical trap which is resisting and you can event measure uh uh the force uh eventually when the motor stalls when it can't move the bead any farther and that's what's called the stall force and that's the maximal force that the motor produces and it's really incredible that one can measure these forces they're one to seven peanes which are very very small forces but they
can be measured quite accurately with an optical trap and uh this is a lot of pioneering work done by Steve block uh and Jim spage in addition one can measure are the steps that are produced by Motors so you can track these single fluorophores that I just showed you with very high resolution um and you can uh see where where how that fluorescent die is moving over time and you can see that that fluorescent die takes abrupt steps uh and these abrupt steps are when the motor is actually moving from one subunit on the track
uh to the next subunit um so the next thing we want to know in order to understand how Motors work is is what they look like what are their structures and for this we need higher resolution techniques like X-ray crystallography and electron microscopy um and we we don't just want one snapshot of the motor we want snapshots of the motor as it goes through its whole atpa cycle it's very much like uh in the in old days when they try to understand how a horse moves they made high-speed cinematography and got various snapshots uh of
the horse in action and that's what we'd like to do with the motor protein we want different snapshots of what that motor looks like at different stages of this atpa cycle now one thing that the structure uh what did tell us right away when we got the structure of kesan is whether the motor protein mein and kinin uh would operate by a similar type of structural mechanism or not before uh the structure was available for Kines we we actually thought that they were completely different types of Motors and there are several obvious reasons one is
that kesan works on microt tibial M works on actin the M motor domain is also over twofold larger than that of Kines and if you asked a computer to line up the sequences the computer said uh that there wasn't any real clear amino acid identity uh on alignment however uh when we got the structure we found a big surprise and that is that there parts of the kinan and M Motors that are very Sim similar to one another structurally in fact these molecular Motors even though they one works on microtubules the other one works on
actin they must have evolved from a common ancestor in some point during Evolution and the part of these motors that's most common is this Central core here which is featured in blue and this is uh does the basic chemistry this is the part that binds the nucleotide it hydes it and it also undergos very small structural changes uh when it's in different nucleotide States for example between ADP and ATP and that mechanism is also very similar between Kines and mein these motors then also have a similar uh what's called a relay Helix which showing here
in green and it relays information from this nucleotide binding site to a mechanical element that I'll describe in a second and it does that by sliding back and forth between the nucleotide side and this mechanical element so these are the mechanical elements of mein and kinesin and indeed those look completely different from one another and they work differently as I'll show you shortly but you can see that these mechanical elements are positioned in the same relative Place relative to this common um enzymatic core here so these two different Motors evolve different kinds of mechanical elements
but they kind of hook them up to the same basic uh enzyme um and sensing unit so now let me tell you a little bit what we've learned from the structure of these motors in different nucleotide States and and how that explains uh the motion of these motors so what you're seeing here is an animation but it's based upon uh real structural data from muscle mein um and this is the actin filament here uh this is this large yellow element is the mechanical element that I just showed you and now let's see how it works
so uh when it IT binds to actin actin causes this phosphate uh group to come off the active site and that causes a large rotation of that big lever arm like unit causing about a 10 nanometer displacement of the actin filament ATP then comes in that dissociates the uh mein and then the hydrolysis Recs that lever arm for another round so here it is again phosphate release that big swing causing the motion the release and the recocking and it's millions and millions of these uh small displacements by masin that collectively result in the contraction of
uh your your muscle and the shortening of that sarir now myosin is made to work in large numbers but kinan has a different problem it has to transport potentially these very small organel that I showed you and uh there's some indication that some of that transport is generated by by single uh motor proteins so kinin has to have uh can't release from the track it has to keep holding on to the track as it moves something that's called processive motility so let me show you how this works so in red here that is the mechanical
element of kinesin uh which is called the neck Linker and I'll show you how that changes its structure during the motility cycle so first the kesan comes onto the microt tual and The microt tual Binding kicks off a bound ADP P ATP can rebind and that ATP binding causes this mechanical element to zipper up along the Blue Core and that as you'll see displaces the partner head so here is the zi ring there is the movement of the partner head from a rear side to a forward site uh and that zippering of the neck Linker
helps to move uh the two motor domains in this hand overand mechanism so here is the zippering and there's the partner head moving from the rear side to a forward s so this this motor protein has learned to kind of walk in a coordinated manner where the two Hand two uh motor domains are moving uh in a leap prog manner along the microt tubio now uh Evolution also has learned to develop different kinds of mechanical elements even within a super family for different purposes so here are two different classes of kinan Motors this is the
one I just showed you in the animation but here is another uh type of member of the kesan super family called kesan 14 and it's learned to walk in the opposite direction of kinan towards the minus end of the microt tual and it's evolved a completely different mechanical element in fact uh it's it's a rigid coil coil structure and we've learned how this mechanical element Works um when it's in its nucleotide free state this this uh uh lever arm which works much more like mein is pointing uh to the plus end but when ATP binds
that lever arm swings and it swings uh its cargo uh and produces motion uh towards the minus end of the microt tual so Evolution has learned to take this basic element of this enzymatic core and couple it on to different mechanical elements to create different types of motility in fact we now know so much about the mechanism of how these motors work that we can start to engineer new kinds of motor proteins with different functions and I'll just give you one example of this this is work from Zeb Bryant's lab but it's a pretty amazing
result where they took that same kinan 14 motor that I just showed you and they added onto that um uh a domain that changes its structure in response uh to Blue Light and I can't give you all the details of it it's found in this paper here but they could design uh this motor such that when the light is switched on the confirmational change uh that's produced upon ATP binding switches the direction of that mechanical element so that it creates movement in the opposite direction of the natural motor and these are microt tubules moving on
glass they're they're marked so you can see the direction and you'll see that they move in One Direction uh in regular light but when you shine blue light on it the motor completely reverses its direction of travel so this just shows and illustrates that you know we can actually begin to engineer these motor proteins ourselves uh now so finally I'd like to end with a discussion a little bit of how these motor proteines are relevant for medicine now um we now know that many uh different diseases are caused by mutations uh in molecular Motors or
in proteins associated with these motors so for example one uh disease which is called familiar hypertrophic cardiomyopathy um is caused by mutations uh in cardiac mein and this is a disease that um is often associated with sudden death in athletes and and this is because they have this um uh enlarged heart due to this uh mutation in this mein mutations and myosin also are associated with deafness uh mutations and dining uh give rise to diseases called ciliary discinesia which have problems with ciliary function such as respiratory dysfunction uh sterility um and and other uh problems
as well and mutations in Kines Motors are associated with neurodegenerative diseases um now the exciting thing is we can also uh modulate uh motor protein function to potentially uh uh amarate certain diseases and I'll give you one example of this uh in uh case of a disease called heart failure where the heart uh fails to contract vigorously enough uh to properly uh pump out uh drugs um and I showed you that that's due to the function of mein cardiac mein uh molecules uh and uh an exciting project that was taken on uh by a company
that I co-founded called cyto kinetics and led actually by my first graduate student fatty Malik was to develop a small molecule that would activate cardiac mein and actually make it perform better to try to improve the contractility of the motors in this failing heart and make it uh make the heart able to pump out blood more effectively and the drug that came out of a lot of work uh is shown here mamp of mabal and we know exactly the biochemical mechanism of this drug it stimulates this one step where uh phosphate is being released uh
by mein and the force producing step uh occurs and this drug stimulates the step so it facilitates entry of mein into the force generating State and this is what uh increases the contractility of the heart so that's just shown here this is an echo cardiogram you're you're looking at um uh the left atrium the left ventricle and the mitro valve and this isn't a patient with heart failure so you can see that this valve is is kind of fluttering a bit and that's due to the poor Contra contractility of the heart in this patient with
heart failure but now after this uh patient is given this drug you can see what happens in this video now you can see that this uh valve is is uh popping up uh much more briskly and that's due to the increased contractility of the heart and this drug uh is now in phase two um uh clinical trials and we'll see if this actually helps these patients with less mortality uh less uh uh less hospitalization and so forth so it's an exciting time and we'll we'll see what happens with this with this drug here so in
this introduction I I I've shown you many things that we know about molecular motor proteins but I want to assure you that there are many open questions and problems to solve um so first of all I describe to you that there are uh tens of motor proteins inside of cells uh and all these motor proteins get hooked up to hundreds of different kind of cargos and we still don't know we know uh in a few cases how this occurs but we don't really know the general rules of what links all these motors onto the correct
caros uh to execute transport functions I also showed you how motor proteins can be tuned to create different kinds of biophysical properties like directionality like velocity uh but we still don't really understand how those bio biophysical Pro uh properties have been tuned by Evolution to create uh very certain types of invivo performance and cellular outcomes and lastly I gave you one example of how we can use uh a drug to modulate a motor uh to treat a particular disease but there probably other ways that we can modulate motor function to uh to treat other kinds
of human diseases as well and that will be a challenge for the future so with this uh I'd like to thank you um and uh in the next uh lecture in this series I'll tell you uh more about our recent research on the dining motor thank you
Related Videos
39:37
Ron Vale (UCSF, HHMI) 2: Molecular Motor P...
Science Communication Lab
205,371 views
30:59
Jared Rutter (U. Utah, HHMI) 1: Mitochondr...
Science Communication Lab
179,585 views
29:37
Nature's Incredible ROTATING MOTOR (It’s E...
SmarterEveryDay
3,013,861 views
24:39
Ron Vale (UCSF, HHMI) 3: Molecular Motor P...
Science Communication Lab
52,922 views

55:59
How the Krebs cycle powers life and death ...
The Royal Institution
351,119 views

37:26
Molecular animation – Tech Talk by Drew Be...
WEHImovies
71,939 views

14:33
Denis Noble explains his revolutionary the...
The Institute of Art and Ideas
342,273 views

20:37
Wallace Marshall (UCSF): Ten Craziest Thin...
Science Communication Lab
194,373 views

1:13:26
Ron Vale - Marvelous Molecular Motors
The TNQ Distinguished Lectures
5,098 views
57:35
The Origin of the Elements
Jefferson Lab
2,713,644 views

52:00
Fungi’s Resilience and Intelligence
Show Me the World
904,129 views
6:21
Your Body's Molecular Machines
Veritasium
4,551,491 views

16:31
The protein folding problem: a major conun...
TEDx Talks
613,328 views

19:16
Ron Vale (UCSF and HHMI): Discovering Kinesin
iBiology Science Stories
15,862 views
9:09
Animations of unseeable biology | Drew Ber...
TED
2,560,718 views

9:34
How NOT To Think About Cells
SubAnima
415,732 views

17:55
We Might Find Alien Life In 7 Years
Veritasium
1,958,437 views

20:15
The 7,800 RPM Motor that Powers Everything...
Clockwork
211,761 views

53:33
Your Brain: Who's in Control? | Full Docum...
NOVA PBS Official
4,365,167 views

1:30:28
Molecular Biology #1 2020
OLLI UCSC
196,166 views