GCSE Chemistry - What is Ionic Bonding? How Does Ionic Bonding Work? Ionic Bonds Explained #14
863.58k views636 WordsCopy TextShare
Cognito
Everything you need to know about ionic bonding!
Ionic bonds form when one atom transfers electron/...
Video Transcript:
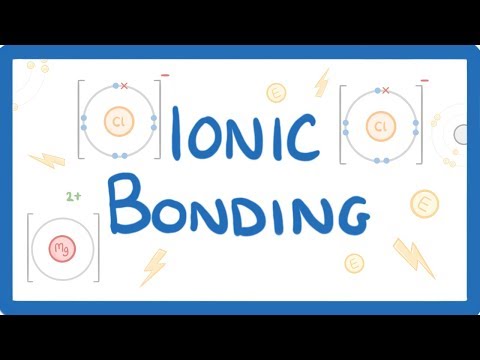
in today's video we're going to take a look at how particles can bond together through ionic bonds and to explain this we'll take a look at some dot and cross diagrams first though i just want to recap what ions are we said in a previous video that ions are formed when atoms lose or gain electrons and we can show this happening with equations for example a sodium atom will go to form a sodium one plus ion plus one electron we know this because if we look at a diagram of a sodium atom it has one
electron in its outermost shell that it needs to lose in order to become stable because remember stability is all about having a full outer shell meanwhile for chlorine we'd write that chlorine plus an electron which we can see it needs to complete its outer shell goes to form a one minus chloride ion now this is all well and good in theory but in real life these reactions don't happen in isolation instead we normally talk about a transfer of electrons from an atom that has too many like sodium to an atom that doesn't have enough like
chlorine once this electron has been transferred both atoms become ions with full outer shells of electrons so we put big square brackets around them and their charge in the top right corner the important bit here is that the two ions have opposite charges so they'll be attracted to each other by electrostatic forces to form an ionic compound we call this force an ionic bond and it's really strong similar in strength to covalent bonds which we cover in another video the way that we've drawn our compound here is known as a dot and cross diagram and
you'll often be asked to draw things this way on your exam to do it properly there are a couple features to notice though one is that we've drawn the electrons of one atom as dots and the other as crosses this is so that we can tell which electrons belong to which atom and you should show the movement of any electrons with an arrow notice that in this dot and cross diagram we've shown every electron shell of the atoms sometimes though you'll be told you only have to draw the outermost shell which is a bit quicker
to draw and for our example it would look like this let's consider a harder example draw the dots and cross diagram for the formation of magnesium chloride mgcl2 only draw the outermost shells now this time we can see that we have three atoms in the compound rather than two to start let's draw out our reactants we have magnesium which has two electrons in this outer shell that it wants to get rid of and we have two chlorines both of which have seven outer electrons so we need one more each the next step is to think
about where the electrons could move to make all the electrons happy with a full outer shell and as a general rule electrons will move from the metal to the non-metal so in this case magnesium can give one electron to each of the two chlorines as a result we'll end up with a magnesium two plus ion and two chloride one minus ions this is now pretty much done however in dotted cross diagrams involving more than two ions we generally arrange the ions like they would be arranged in a real compound so because the chlorides will both
be attracted to the positive magnesium we place them on either side of it and that's it for this video if you enjoyed it then do share with your friends and we'll see you next time
Related Videos

6:08
GCSE Chemistry - What is an Ionic Compound...
Cognito
496,971 views

5:33
GCSE Chemistry - Covalent Bonding #16
Cognito
846,440 views

J.E.D.I. Training VERBAL for DIGITAL SAT -...
Scalar Learning
6:24
GCSE Chemistry - Electron Arrangement #8
Cognito
346,142 views

11:50
Types of Bonding (Ionic, Covalent, Metalli...
Miss Wetton - GCSE Science Revision
433,402 views

4:29
GCSE Chemistry - The Mole (Higher Tier) #25
Cognito
743,158 views
12:50
Introduction to Ionic Bonding and Covalent...
The Organic Chemistry Tutor
2,076,428 views
12:23
Covalent vs. Ionic bonds
Beverly Biology
1,004,975 views

4:45
GCSE Chemistry - Metals and Non-Metals #10
Cognito
470,272 views

3:31
GCSE Chemistry - Metallic Bonding #20
Cognito
655,628 views

7:26
Lewis Diagrams Made Easy: How to Draw Lewi...
ketzbook
4,743,931 views
23:55
Bonding (Ionic, Covalent & Metallic) - GCS...
Science Shorts
691,213 views

4:49
GCSE Chemistry - Halogens and Noble Gases ...
Cognito
432,225 views

2:32
Cations Vs. Anions: A Simple Guide To Unde...
Tadashi Science
35,010 views
4:46
GCSE Chemistry - Properties of Simple Mole...
Cognito
445,151 views
5:35
GCSE Chemistry - Fractional Distillation a...
Cognito
639,375 views

7:06
GCSE Biology - What are Nutrients? Carbohy...
Cognito
239,745 views