Electrochemistry
312.37k views759 WordsCopy TextShare

Professor Dave Explains
How does a battery work? Now that you think about it, you have no idea, do you? Well take a gander! ...
Video Transcript:
professor Dave here let's talk about electrochemistry. this should be a familiar object to everyone but how do batteries work? the first one was invented by Alessandro Volta in 1800 and the chemistry hasn't changed too much since then.
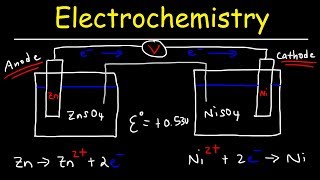
the chemistry occurring in a voltaic cell is just a spontaneous oxidation-reduction reaction which we now know is a transfer of electrons. the only difference is that the oxidation and reduction half reactions are physically separated from one another. the anode is where the oxidation takes place and the cathode is where the reduction takes place electrons captured in the oxidation then flow from the anode to the cathode which generates a current of electrical energy which can be harvested to do work.
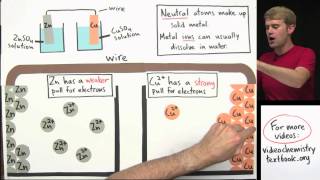
each side of the cell is called a half cell and they contain ions in solution. there is also something called a salt bridge from which ions flow to maintain charge balance at the anode neutral zinc atoms give up two electrons to become zinc ions which then fall off the electrode into solution. at the cathode copper ions in solution near the electrode gain two electrons to neutralize and join the copper lattice.
ions in the salt bridge compensate for this gradual change in overall charge a voltaic or galvanic cell is one example of a kind of electrochemical cell in which a spontaneous oxidation reduction reaction generates an electric current. in an electrolytic cell an electric current drives an otherwise non spontaneous reaction. when we describe the components of a voltaic cell we have to use a particular notation.
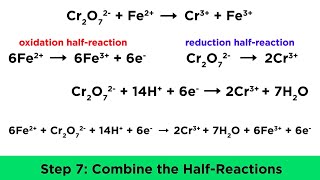
we list the oxidation half cell or the anode on the left and the reduction half cell or the cathode on the right. the double vertical lines represent the salt bridge that separates the half cells and single vertical lines represent phase boundaries like between a solid electrode and ions in solution. we can go between this electrochemical notation and the balanced cell reaction by just including the number of electrons involved in each half reaction then use the least common multiple of electrons to combine the half reactions into the cell reaction and electrons will cancel.
let's try to understand the concept of electron flow just as water flows from high pressure to low pressure electrons flow from areas of high to low electric potential. so electric potential is kind of like electric pressure. potential difference is the difference in electric potential between two points in the case of a voltaic cell those points would be the anode and the cathode and their potential difference is called the cell potential or Ecell, this will be expressed in volts.
for a voltaic cell we measure the cell potential by looking at the oxidation potential and the reduction potential but since oxidation and reduction are reverse process these will simply be the opposite of each other so we arbitrarily decided to tabulate reduction potentials and Ecell for a voltaic cell will be Ecathode minus Eanode because we are measuring the reduction that happens at the cathode and then adding the reduction that could happen at the anode but reversing the sign because it is actually oxidation that is taking place so we have a whole list of reduction potentials and these numbers tell us how favorable it is for a substance to be reduced we can see here that fluorine is a very good oxidizing agent as it has a high reduction potential which makes sense since fluorine has a very high electron affinity. on the other end elements like lithium and sodium have very negative reduction potentials making them good reducing agents since they have a tendency to be oxidized as it is more favorable for them to lose an electron than to gain one. to calculate Ecell for any hypothetical voltaic cell containing two of these substances just calculate Ecathode minus Eanode the larger the Ecell value the greater the current the cell will generate the change in Gibbs free energy for the cell can be related to the cell potential, this is the maximum work of a voltaic cell is capable of.
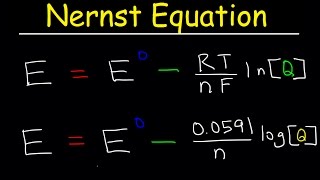
here n is the moles of electrons exchanged in the reaction and F is the Faraday constant the electric charge of a mole of electrons. we can also relate cell potential to its standard cell potential under standard conditions by using the Nernst equation.
Related Videos
14:12
Nuclear Reactions, Radioactivity, Fission ...
Professor Dave Explains
861,730 views
27:42
Introduction to Galvanic Cells & Voltaic C...
The Organic Chemistry Tutor
710,826 views

16:37
Introduction to Electrochemistry
Tyler DeWitt
1,839,885 views
9:04
Electrochemistry: Crash Course Chemistry #36
CrashCourse
2,229,396 views

35:04
The Problem With Sabine Hossenfelder
Professor Dave Explains
714,636 views

3:52
Oxidation-Reduction Reactions
Professor Dave Explains
791,149 views

1:27:17
Electrochemistry Review - Cell Potential &...
The Organic Chemistry Tutor
966,188 views

32:46
Electrolysis
Tyler DeWitt
2,526,104 views
13:00
Electrolytic vs Galvanic (Voltaic) Cell | ...
Leah4sci MCAT
2,604 views
30:53
Nernst Equation Explained, Electrochemistr...
The Organic Chemistry Tutor
645,800 views

27:14
19.4 How to Calculate Standard Cell Potent...
Chad's Prep
40,811 views

32:18
No, Sabine, Science is Not Failing
Professor Dave Explains
393,079 views
7:31
Balancing Redox Reactions in Acidic and Ba...
Professor Dave Explains
999,070 views
23:35
Galvanic Cells (Voltaic Cells)
Tyler DeWitt
1,838,098 views
53:39
Electrochemistry Practice Problems - Basic...
The Organic Chemistry Tutor
212,611 views
10:56
Cell Potential Problems - Electrochemistry
The Organic Chemistry Tutor
749,488 views
![Electrochemical Cells [IB Chemistry SL/HL]](https://img.youtube.com/vi/0NY_L9u7Hkw/mqdefault.jpg)
14:00
Electrochemical Cells [IB Chemistry SL/HL]
Revision Village
1,382 views

13:08
We Fell For The Oldest Lie On The Internet
Kurzgesagt – In a Nutshell
4,366,089 views

20:47
How Physicists Broke the Solar Efficiency ...
Dr Ben Miles
691,253 views