Proteins
1.54M views2014 WordsCopy TextShare
Bozeman Science
Paul Andersen explains the structure and importance of proteins. He describes how proteins are crea...
Video Transcript:
[Music] hi it's Mr Anderson and in this video I'm going to talk about proteins proteins are incredibly important because that's what we're made up of when you look at me you're looking at my proteins we just completed the Human Genome Project and we figured out the DNA in a typical human but now we're headed into What's called the proteome project where we try to figure out what are these proteins made up of and what do they look three-dimensionally this used to be incredibly hard process this is John kendrew here putting together a model for myoglobin
and you had to do it by hand we now do a lot of this with computers um but you can help at the end of this video I'm going to show you a program called fold it and you can actually build and fold proteins that are going to be used in scientific research and so it's a really cool thing and it's a really cool time in reference to proteins but let's start by building a little bit of knowledge proteins are made of amino acids and most seventh graders understand this they're the building blocks of proteins
but where do we get those amino acids we get them in our diet and so basically we eat proteins we break them down into amino acids then we can weave those back together again into the proteins that make us and so when you're looking at me the amino acids in my skin used to be part of my food and so I literally am what I eat now here's five different amino acids there are a total of 20 that we used in life but here's five basic amino acids I when to show this to um my
students they tend to get a little bit overwhelmed because there's too much chemistry here on one page but let's try to figure out the things that are the same on this page and so basically if we were to look at the middle of each of these we find that there's a carbon with a hydrogen attached to it we call this carbon the alpha carbon it's going to sit right in the middle what else is the same in every amino acid well on the left side we have a nitrogen attached to two hydrogens we call this
an amino group and then if we look on the right side we have what's called a carboxy group and so basically every Amino acidd is going to have these three similar parts and so the only thing that's going to be different is going to be what comes off the bottom and we call that the R Group uh and so all amino acids are the same except what comes off here and that gives it different properties and so let's kind of see how they're put together and so when I build proteins inside my body I'm doing
that with amino acids and we use that something called a dehydration synthesis to do that and so let me move this one over here basically what we do as we position one amino acid right next to an amino acid you can see here that there is two hydrogens and an oxygen here and you know that in chemistry if we have two hydrogens H2O that's simply a water and so what we can do is we can lose that water now it's not as simple as that this whole thing sits inside a ribosome so there's a giant
enzyme around the outside of it but let's attach another one so now we bring another one right next to it you can see that the hydroxy group of the carboxy group is attaching into the hydrogen we're going to lose a water and then we're going to form another Cove valent Bond and then we put another one next to it we lose another water we form a a covalent bond and we do that again and now we have what's called a polypeptide so each of these individually are called a peptide but if we attach them all
together we have a polypeptide and you can see that this strand across the top looks the same um it looks uniform but the only thing that's going to be different in each of these is going to be the r group that Trails off the bottom so where does this occur well basically these are the amino acids this would be the ribosome in a cell and all life does this basically you have these little trnas that'll bring their amino acids in and then we attach them together and when you have a bunch of amino acids attached
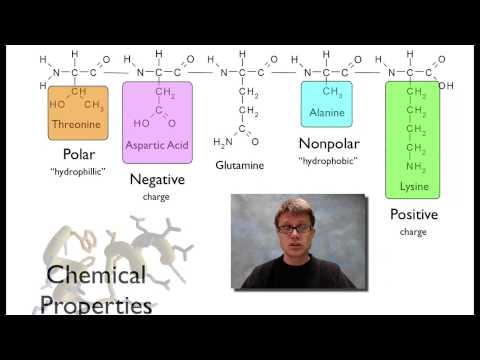
together you have a polypeptide and that polypeptide will eventually fold into a protein and so here's our five amino acid sequence right here these are each going to have different chemical properties and so for example this one right here threonine is going to be polar what that means is it's going to have a charge if we look at alanine right down the way this is going to be non-polar and so why is that important well if you're polar then you're hydrophilic that means that water's going to be attracted to you in other words we're going
to find threonine in the presence of water but alanine since it's got this methyl group right here it's not going to be attracted to the water and so it's going to hide from the water or if we look at the aspartic acid it's going to have a negative charge and if we look at the lysine over here it's going to have a positive charge and so these two things uh or these two amino acids are found in the same polypeptide they're going to try to get next to each other because the positive and the negative
are going to attract and so what you end up getting is a three-dimensional protein the middle part so this brownish tan part in the middle is going to be the backbone and that's going to be again made up of all of the parts of the amino acid that are the same so the amino the Alpha and the carboxy group over and over and over again but all these things on the outside that are trailing off are going to be the r groups these are going to be these residue groups that kind of fold off the
end and so this right here would be a polypeptide this would be a number of different amino acids attached together and these usually have thousands of amino acids in a typical protein like hemoglobin would be an example of one that's found in your blood and so basically this will fold into a three-dimensional shape and what I tell students is a polypeptide is just going to be the sequence of those amino acids and once it's folded into a specific shape then we can call it a protein and so the first you've Maybe read this there are
four levels of structure in a protein and so let me talk you through that first and then I got a little model that'll hopefully help and so the primary structure is going to be the order that those amino acids are bonded together the next thing we have are what are called Alpha hoses and beta pleated sheets and we call that the secondary structure and so Helix looks like this a beta pleated sheet is going to be two sides that are attached to each other and these little dots in the middle are going to be hydrogen
bonding between adjacent Sid sides of that polypeptide and so this is the structure it comes out first it's going to be linear next we have the alpha helices the beta pleed sheets then we have the tertiary structure tertiary or the third level of structure is going to be all those R groups interacting and so maybe we have one that's hydrophobic it'll hide to the middle or hydrophilic on the outside we'll have D sulfide bonds we'll have positive attracting to negative but this is the third level of structure and then finally we have quatron structure maybe
we have this one polypeptide together or this protein together with another protein so hemoglobin is an example of that made of a number of different subunits so here's my little model and so if you look at this model you can think of this being a polypeptide so it's made up of amino acid after amino acid and then it's going have all the r groups on the underside those are going to be the only things that are different all these R groups coming off the bottom and so basically primary structure like this secondary structure is going
to be the Alpha helices and the beta pleated sheets and so an alpha will look like this what's holding that in place is simply going to be the hydrogen bonds and then we're going to have a beta pleated sheet a beta pleated sheet might look something like that so there's going to be hydrogen bonds between here and here but maybe this right here is a real Hydra phobic R Group and it's going to fold right to the middle and then this might be hydrophilic it's going to fold to the outside we might have positive attracted
to negative and then we eventually have a three-dimensional shape of a protein now this may combine with other proteins but what's cool about proteins is their structure fits their function if it doesn't have the structure if we heat it up if we cool it if we change the acidity basically it'll fold apart we call that Den nature and then it doesn't work anymore and so I said at the end that you could help in science and so there's a program called fold it I'm going to launch it and I'll be back in just a second
and so in this program what you're given is a simple polypeptide so we have two amino acids and then this is going to be the R Group and what you do in this video game is you try to make the r groups happy and so I've already cleared level one so let's go on to level two next puzzle you can download this for Mac Linux and windows let me quickly turn this one around you can see here that we have a couple of let me get the help out of the way you have a couple
of different amino acids and then there R groups I can pull those apart and they're going to be a little bit happier and then I can clear the level and so what are you really doing as I play this game I could talk basically what you're doing is learning the rules of protein folding but these problems are going to get harder and harder and harder you can see an alpha Helix here and so what you can do is you can get really good at folding these proteins and what's neat about this is people are playing
folded hour after hour after hour and it hit the news last year where a c a couple of protein folders probably a team of protein folders decoded the shape of a really important enzyme and HIV infection and so it's plausible that in the future gamers are going to win a Nobel surprised for the work they do on protein folding because we know the primary structure of proteins but we don't have any idea of how the three-dimensional shape is put together and computers are good at this but it turns out that humans are maybe a little
bit better and so those are proteins uh and I hope that was helpful
Related Videos

8:00
Nucleic Acids
Bozeman Science
825,424 views

15:20
Biological Molecules
Bozeman Science
1,004,357 views

51:40
3. Structures of Amino Acids, Peptides, an...
MIT OpenCourseWare
234,734 views

27:15
The Most Misunderstood Concept in Physics
Veritasium
21,830,786 views

22:19
Physicist Brian Cox explains quantum physi...
Big Think
1,517,038 views

8:49
Carbohydrates
Bozeman Science
897,968 views
10:50
Protein Structure
Professor Dave Explains
1,341,730 views

17:49
Marine Biologist Answers Fish Questions Fr...
WIRED
3,826,147 views

16:34
The Forgotten Prehistoric War That Killed ...
ExtinctZoo
3,514,226 views
10:47
The Molecules of Life
Bozeman Science
944,899 views

11:57
Transcription and Translation
Bozeman Science
1,861,223 views

27:14
Transformers (how LLMs work) explained vis...
3Blue1Brown
6,220,797 views

23:58
Memorize The 20 Amino Acids - The Easy Way!
The Organic Chemistry Tutor
1,048,187 views
11:52
Enzymes
Bozeman Science
2,049,758 views

12:29
DNA and RNA - Part 1
Bozeman Science
729,000 views
7:46
Protein Structure and Folding
Amoeba Sisters
2,666,842 views

27:03
Schiff Takes To Senate Floor to Lay Out Tr...
Sen. Adam Schiff
2,860,026 views

5:35
Trump Middle East Trip Cold Open - SNL
Saturday Night Live
3,979,182 views

1:13:44
What Bothers Physicists About Black Holes ...
Cleo Abram
1,169,194 views